† Corresponding author. E-mail:
We put forward a two-step route to synthesize vanadium diselenide (VSe2), a typical transition metal dichalcogenide (TMD). To obtain the VSe2 film, we first prepare a vanadium film by electron beam evaporation and we then perform selenization in a vacuum chamber. This method has the advantages of low temperature, is less time-consuming, has a large area, and has a stable performance. At 400 °C selenization temperature, we successfully prepare VSe2 films on both glass and Mo substrates. The prepared VSe2 has the characteristic of preferential growth along the c-axis, with low transmittance. It is found that the contact between Al and VSe2/Mo is ohmic contact. Compared to Mo substrate, lower square resistance and higher carrier concentration of the VSe2/Mo sample reveal that the VSe2 film may be a potential material for thin film solar cells or other semiconductor devices. The new synthetic strategy that is developed here paves a sustainable way to the application of VSe2 in photovoltaic devices.
Transition metal dichalcogenides (TMDs) materials, which exhibit unique structural features and intriguing properties, have attracted much attention recently.[1–4] As a typical transition metal dichalcogenide, vanadium diselenide (VSe2) is made up of Se–V–Se layers.[5,6] Compared to the semiconducting MoSe2 and other TMDs, VSe2 is metallic in nature due to the strong electron coupling interaction for all neighboring V4+–V4+ pairs.[7] In addition, VSe2 shows intrinsic ferromagnetism behavior[8,9] and extra-high electrical conductivity.[10]
However, most of the VSe2 reports are related to their electromagnetic properties, and its application in optoelectronic devices is rare. The main challenge limiting the application of VSe2 in this respect is the limitation of its preparation method. Over the past few decades, many methods to prepare VSe2 have been suggested. The VSe2 was first prepared in the tube furnace at high temperature.[11,12] At present, the synthesis method of chemical vapor transport (CVT) usually requires a long reaction time (72–120 h) and a high reaction temperature (800-900 °C).[13–15] Compared with other methods, the chemical vapor deposition (CVD) method should be the best choice for preparing the high crystalline quality VSe2.[10,16–20] However, it also needs high reaction temperature and long reaction time. To prepare VSe2 at low temperature, aqueous solution was used to synthesize the bulk VSe2 for the first time by Xu et al.[6] Other groups have proposed different chemical-based methods to synthesize VSe2, such as one-pot solvothermal method,[21] hydrothermal method,[7,22,23] and one-pot colloidal method.[5] However, these wet methods of preparing VSe2 have not been widely used because of the complex chemical environment in solution.
Herein, we put forward a two-step method to prepare VSe2 films. This method has the advantages of low temperature, large area, and stable performance. Simultaneously, the reaction time is very short. In our synthesis, non-toxic vanadium metal column and Se powder are used as the metal sources. In the first step, we use electron beam evaporation to obtain a vanadium film. In the second step, the vanadium film is selenized in a closed vacuum chamber. We not only successfully prepare VSe2 films on the glass substrates, but also obtain dense VSe2 films on the Mo substrates. This two-step process for preparing VSe2 proposed in this paper can be compatible with the fabrication process of many optoelectronic devices; for example, VSe2 can be used as a back contact layer between CdTe or CZTSSe and back electrodes.[24,25]
The two-step synthesizing procedure of VSe2 flakes is schematically depicted in Fig.
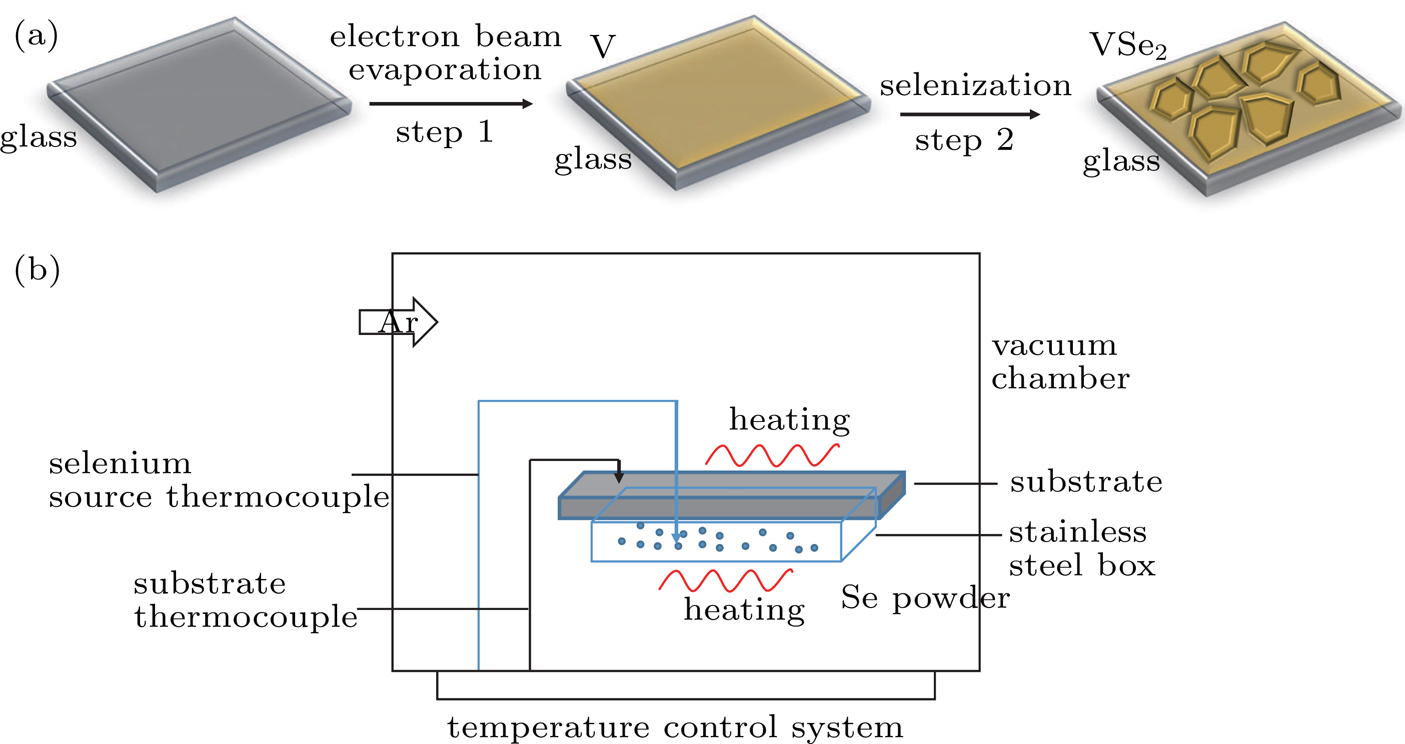 | Fig. 1. (a) Schematic diagram of the synthesization of the VSe2 film with two-step method, (b) schematic diagram of the vacuum chamber for selenization process. |
The specific selenization process is shown in Fig.
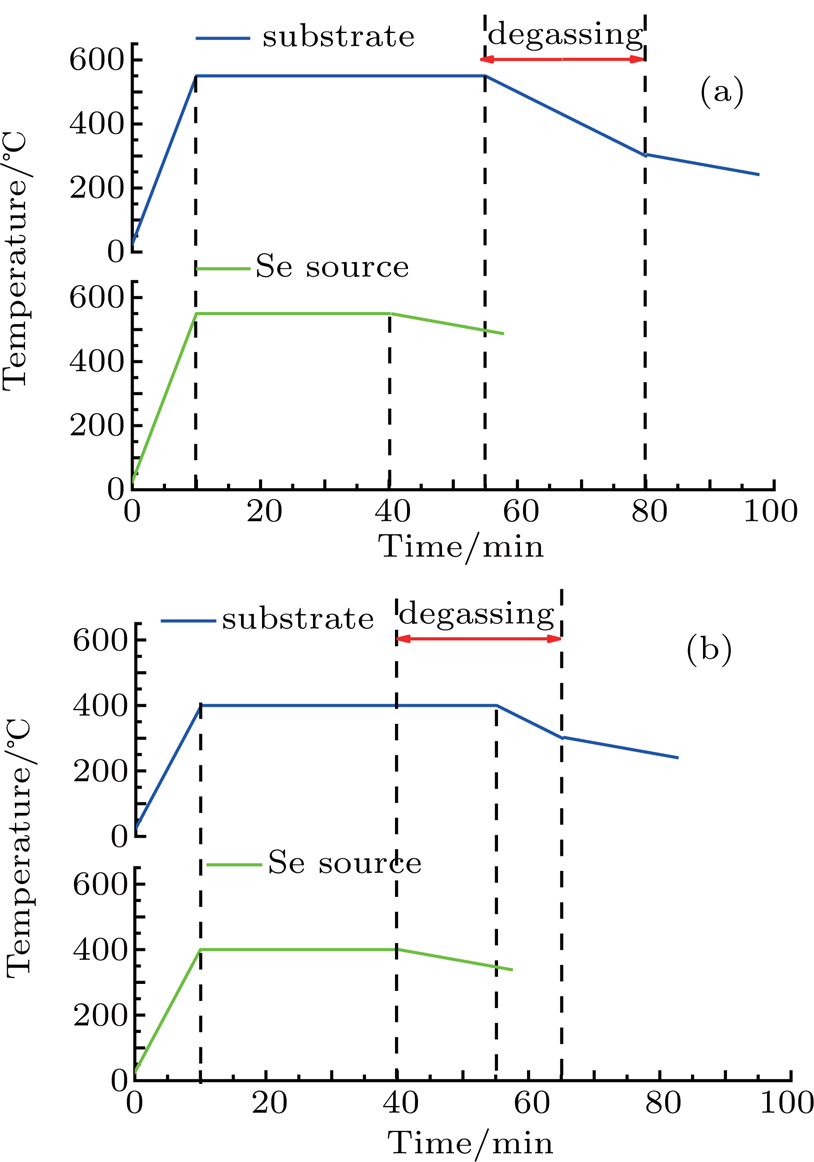 | Fig. 2. (a) Selenization process at a selenization temperature of 550 °C and (b) at a temperature of 400 °C. |
The phase structures of the films were measured by the PANalytical X’Pert pro x-ray diffractometer (XRD), which used Cu-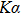


Figure 
This experiment indicates that the selenization temperature is high. Then the selenization temperature was decreased to 400 °C. To prevent the appearance of Se on the surface of the film, the chamber is degassed as soon as the Se cools down, as shown in Fig.
 | Fig. 4. XRD patterns and SEM images of the samples with (a), (c) 50 nm and (b), (d) 100 nm thick vanadium film selenized at 400 °C. |
According to these results, VSe2 can be prepared by selenizing 50 nm-thick V film at 400 °C. Then 50 nm thick V films were selenized at different temperatures to study the VSe2 film growth behavior. Figure
 | Fig. 5. XRD patterns of the V films selenized at (a) 350 °C, (b) 400 °C, (c) 450 °C, and (d) 500 °C, the inset shows the corresponding SEM images of the films. |
X-ray photoelectron spectroscopy (XPS) is carried out to further investigate the composition of the sample. The binding energies of 517.0 eV and 524.0 eV correspond to V 2p1/2 and 2p3/2, which indicate the V4+ state, as show in Fig.
 | Fig. 6. XPS spectra of (a) V 2p and (b) Se 3d regions for the sample of the 50 nm thickness V films selenized at 400 °C. |
Optical properties of the as-grown thin films selenized at different temperatures are shown in Fig.
If VSe2 is used in a solar cell, such as the kesterite structured solar cell, then it will be grown on Mo back contact film. Considering that the excessive thickness of the VSe2 layer deteriorates the performance of the device, we chose a 50 nm thick V film for research instead of 100 nm. Thus, we prepared a 50 nm thick V film on a Mo substrate. Both Mo and V films are preferentially grown in the (110) direction (Fig.
The V film prepared on Mo substrate was then selenized at the temperature of 400 °C. Figure 
To understand the contact properties between Mo (metal) substrate and VSe2 (semiconductor material), we prepared a certain thickness of Al electrode on the Mo surface and VSe2 surface, and then performed the dark state I–V test. In Fig.
In addition, we also performed a Hall test on the above samples. The test results are summarized in Table
| Table 1.
The Hall measurements of Mo substrate and VSe2 films prepared on Mo substrate. . |
In summary, we demonstrate a new two-step route to synthesize the VSe2 film. By comparing the different selenization temperatures, we find that the VSe2 film has the best crystal quality when the selenization temperature is 400 °C. At 400 °C selenization temperature, we can stably prepare VSe2 film on both glass and Mo substrates. The XRD patterns of the VSe2 film indicate the VSe2 preferential growth along the c-axis. A number of hexagonal nanosheets can be clearly seen from the SEM image. Moreover, the sample of V thin film selenized at 400 °C has the highest reflectivity and the lowest transmittance. This indicates that VSe2 is well suited for application in optoelectronic devices. In addition, the contact between Al/VSe2/Mo belongs to ohmic contact, and the lower square resistance of VSe2/Mo sample can be observed. VSe2 is a potential material for solar cells. Moreover, this work opens up the opportunity for extensive research on the application of VSe2 in photovoltaic device or other semiconductor material devices.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] |








