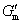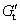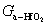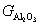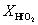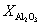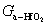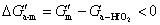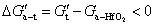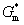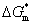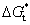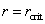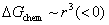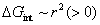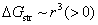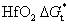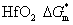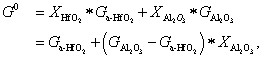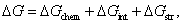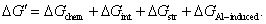1. IntroductionRecently, post high-k gate dielectric materials with superior properties to those of the conventional high-k materials have been studied extensively in advanced gate-stack structures in further scaled metal-oxide–semiconductor field-effect transistor (MOSFET).[1–5] In general, the dielectric constants of high-k materials can be affected by their degrees of crystallinity, crystal structures, and crystallographic orientations, in addition to their stoichiometric compositions. In the case of HfO2, it possesses four crystalline phases (monoclinic, tetragonal, cubic, and orthorhombic), and the cubic and tetragonal high-temperature hafnia phases show much higher dielectric constants (on the order of 30–40) than the monoclinic and amorphous phases.[6–8] There have been many reports showing that the dielectric constant of HfO2 can be enhanced by doping Y2O3, Al2O3, SiO2, etc. due to the change in the crystal structure of the film.[9–16] However, these studies have typically relied on films with thickness values larger than 10 nm, few articles have been published which strived to understand how the dielectric constant is related to the crystal structure of ultrathin (<5 nm) HfO2 film.
In this letter, we report an experimental result based on phase transition engineering that a great deal of tetragonal phase HfO2 can be stabilized in the ultrathin (about 4-nm thick) HfO2 film when being annealed at temperatures above 650 °C due to the addition of small concentration Al atoms into HfO2 film. In addition, we study the phase transition behavior of ultrathin Al-doped HfO2 film from the viewpoint of thermodynamics and kinetics for the first time. A model for the total Gibbs free energy is proposed to help to understand the physical mechanism behind.
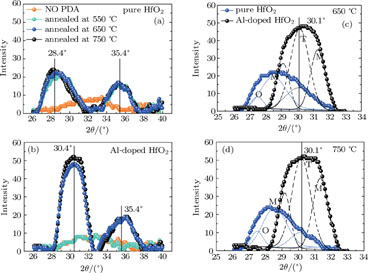
3. Results and discussionThe crystallinities of the pure HfO2 and Al-doped HfO2 film before and after annealed at temperatures ranging from 550 °C to 750 °C are investigated by GIXRD as shown in Fig. 1. The x-ray wavelength is 0.154 nm and the x-ray beam is incident at a grazing angle of 0.2°. As shown in Fig. 1(a), which displays the GIXRD spectra in a 2θ range of 26°–39° for the pure HfO2 films, the GIXRD pattern of as-deposited pure HfO2 film shows no peak, reflecting its amorphous nature, while the profile of pure HfO2 film annealed at 550 °C–750 °C shows two peaks around 28.4° and 35.4°. Figure 1(b) displays the GIXRD spectra in a 2θ range of 26°–39° for the Al-doped HfO2 films annealed at different temperatures. The as-deposited Al-doped HfO2 film is also amorphous. However, compared with the GIXRD pattern of the pure HfO2 film annealed at 550 °C, the profile of Al-doped HfO2 film annealed at 550 °C shows no peak, indicating that the incorporation of Al2O3 can increase the thermal stability of HfO2 film, which has been reported in previous literature.[19–21] The profiles of Al-doped HfO2 films annealed at 650 °C and 750 °C also show two peaks, the first peak is located around 30.4° and the second one is located around 36°. Interestingly, compared with the first peak of pure HfO2 film, the peak value and intensity of the Al-doped HfO2 film are obviously increased. We find that the intensity of the peak in a 2θ range of 26°–33° originates from three partial overlapped peaks with Gaussian shapes. As shown in Figs. 1(c) and 1(d), the three peaks of pure HfO2 film are located at 27.5°, 28.4°, and 30.1° corresponding to orthorhombic phase (O), monoclinic phase (M), and tetragonal phase (T), respectively. It can be concluded that the pure HfO2 films annealed at 650 °C and 750 °C are composed of orthorhombic, monoclinic and tetragonal phase, among which the monoclinic phase is dominant. While the three peaks of Al-doped HfO2 film are located at 29.1°, 30.1° (T), and 31.3° (M). We think, the peak located at 29.1° is related to HfxAlyO. Compared with the pure HfO2 film, the Al-doped HfO2 film has three peaks whose intensities are increased. Moreover, the strongest peak intensity is observed for the tetragonal phase, indicating that the tetragonal phase is dominant in each of the Al-doped HfO2 films annealed at 650 °C and 750 °C, which is different from the scenarios of pure HfO2 films. Additionally, there are only slight increases in the peak intensity for all films with the annealing temperature increasing from 650 °C to 750 °C due to the ultrathin film thickness. According to the GIXRD results, we conclude that the phase transition temperature of hafnia is reduced due to the addition of ~ 4% Al atoms. Especially for the tetragonal phase, which was reported to be about 1700 °C for thick hafnia in Ref. 22. In other words, the Gibbs energy barrier for the nucleation of crystallographic hafnia phase is reduced from the viewpoint of kinetics, and the Gibbs energy barrier for the tetragonal phase is smaller than that for the monoclinic phase. In addition, the stabilization of metastable tetragonal HfO2 phase by adding ~ 4% Al atoms indicates that the Gibbs free energy of the tetragonal HfO2 phase is reduced approximately to the Gibbs free energy of the monoclinic HfO2 phase from the viewpoint of thermodynamics.
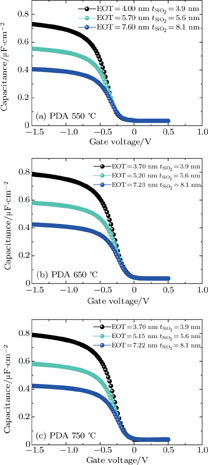
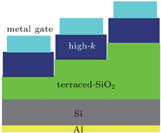
The experimental MOS structure with 4-nm Al-doped HfO2 film and terraced thickness SiO2 film as dielectric film is shown in Fig. 2, the Al-doped HfO2 thickness is fixed at 4 nm because the purpose of this study is to examine the ultrathin (about 4 nm) Al-doped HfO2 film properties. Figures 3(a)–3(c) are the C-V curves of MOS capacitors with dielectric films annealed at 550 °C, 650 °C, and 750 °C, respectively. The equivalent oxide thickness (EOT) is extracted from the accumulation capacitance.
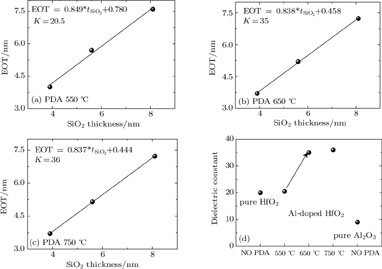
Figures 4(a)–4(c) show the relationship plots between the SiO2 physical thickness and the equivalent oxide thickness (EOT) of MOS capacitors with dielectric films annealed at 550 °C, 650 °C, and 750 °C, respectively. Then, the dielectric constant of each film can be determined from the intercept of its plot. Figure 4(d) clearly shows that Al-doped HfO2 films annealed at 650 °C and 750 °C each have a significant enhancement of dielectric constant over pure HfO2 despite the fact that Al2O3 has a lower permittivity (~9) than pure HfO2 (~ 20). This can be attributed to the fact that a great number of tetragonal phases are formed in the film annealed at 650 °C, which has a higher dielectric constant than amorphous and monoclinic phase. When the annealing temperature reaches 750 °C, the dielectric constant increases very slowly (~ 36) due to the limitation to the number of the tetragonal phases, which is attributed to the small Al atom concentration and ultrathin HfO2 film thickness. The dielectric constant of the Al-doped HfO2 film annealed at 550 °C keeps almost unchanged, which can be accounted by the fact that the film remained amorphous at 550 °C. The dielectric constant behavior is consistent with the GIXRD results discussed above.
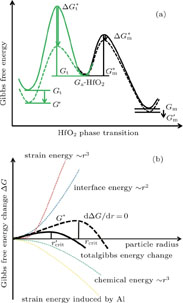
In general, crystallization is dominated by both kinetic and thermodynamic factors. Figure 5(a) shows the Gibbs free energy schematic diagram of the phase transition of Al-doped HfO2 film. Gm and Gt represent the Gibbs free energies of monoclinic and tetragonal phase of pure HfO2, and 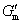 and
and 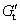 represent the Gibbs free energies of monoclinic and tetragonal phase of Al-doped HfO2 film annealed at 650 °C, respectively.
represent the Gibbs free energies of monoclinic and tetragonal phase of Al-doped HfO2 film annealed at 650 °C, respectively. 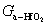 and
and 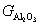 represent the Gibbs free energies of amorphous pure HfO2 and Al2O3, and
represent the Gibbs free energies of amorphous pure HfO2 and Al2O3, and 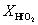 , and
, and 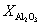 represent the atomic mole percentages of HfO2 and Al2O3 in the Al-doped HfO2 film, about 96% and 4% respectively. G0, the total Gibbs free energy of as-deposited Al-doped HfO2, is approximately equal to
represent the atomic mole percentages of HfO2 and Al2O3 in the Al-doped HfO2 film, about 96% and 4% respectively. G0, the total Gibbs free energy of as-deposited Al-doped HfO2, is approximately equal to 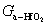 as given in Eq. (1). So
as given in Eq. (1). So 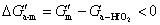 and
and 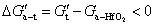 can be taken as the thermodynamic driving force for the phase transition of Al-doped HfO2 from amorphous to crystallization. On the way of transiting HfO2 phase from amorphous to either monoclinic or tetragonal phase, the system need to overcome the Gibbs energy barrier by thermal activation.
can be taken as the thermodynamic driving force for the phase transition of Al-doped HfO2 from amorphous to crystallization. On the way of transiting HfO2 phase from amorphous to either monoclinic or tetragonal phase, the system need to overcome the Gibbs energy barrier by thermal activation. 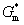 and
and  represent the Gibbs energy barriers for the nucleation of pure HfO2 from amorphous to monoclinic and tetragonal phase respectively.
represent the Gibbs energy barriers for the nucleation of pure HfO2 from amorphous to monoclinic and tetragonal phase respectively. 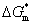 and
and 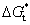 represent the decreases of the Gibbs energy barrier for the nucleation of Al-doped HfO2 from amorphous to monoclinic and tetragonal phase respectively.[23–24] Figure 5(b) describes the variations of the Gibbs free energy of a growing spherical new phase particle with particle radius r. If a particle with
represent the decreases of the Gibbs energy barrier for the nucleation of Al-doped HfO2 from amorphous to monoclinic and tetragonal phase respectively.[23–24] Figure 5(b) describes the variations of the Gibbs free energy of a growing spherical new phase particle with particle radius r. If a particle with 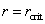 occurs, the system can lower its Gibbs energy spontaneously with the new phase particle growing. Considering the formation of a spherical new phase (monoclinic or tetragonal) particle of radius r in amorphous pure HfO2 film, the total Gibbs energy change ΔG can be given as Eq. 2.
occurs, the system can lower its Gibbs energy spontaneously with the new phase particle growing. Considering the formation of a spherical new phase (monoclinic or tetragonal) particle of radius r in amorphous pure HfO2 film, the total Gibbs energy change ΔG can be given as Eq. 2. 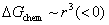 is the change in chemical energy driving the transformation.
is the change in chemical energy driving the transformation. 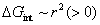 is the change in interface energy associated with the occurrence of interface between new phase and parent phase opposing the transformation.
is the change in interface energy associated with the occurrence of interface between new phase and parent phase opposing the transformation. 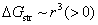 is the change in strain energy associated with the volume expansion opposing the transformation.[23] Similarly, the total Gibbs energy change ΔGʹ of Al-doped HfO2 film can be given as Eq. 3. Compared with Eq. 2, equation 3 has the fourth term
is the change in strain energy associated with the volume expansion opposing the transformation.[23] Similarly, the total Gibbs energy change ΔGʹ of Al-doped HfO2 film can be given as Eq. 3. Compared with Eq. 2, equation 3 has the fourth term  . After 550 °C annealing, Al atoms remain in Al2O3 layer because there is no enough thermal energy to break down Al–O bond, and the incorporation of Al2O3 can increase the thermal stability of HfO2 film as reported before. After 650 °C annealing, Al atoms will diffuse from Al2O3 layer into HfO2 layer and substitute Hf atoms thus causing additional strain energy ΔGAl−induced associated with the local lattice distortion. As the result of thermal fluctuation and by chance, a local atomic rearrangement will occur such that a new phase particle is created compatible to the thermodynamically preferred phase. The formation of new phase around Al atoms can gradually release part of the strain energy with the particle growing. It follows from Fig. 5(b) that ΔGAl−induced can result in a decrease in the nucleation barrier G* for either monoclinic or tetragonal phase HfO2. On the other hand, for alumina, the Al coordination number has a value between 4 and 6, and the Al–O bond lengths are between 1.71 Å and 1.79 Å.[25] For monoclinic phase HfO2, the Hf coordination number is seven and the average Hf−OI and Hf−OII bond lengths are 2.086 Å and 2.197 Å, respectively.[26] For tetragonal phase HfO2, the Hf coordination number is eight and the Hf–O bond lengths are equally distributed at 2.07 Å and 2.37 Å.[27] Obviously, there are four shorter Hf–O bonds in tetragonal phase HfO2 that will facilitate the formation of Al–O bonds with relatively small lattice distortion. In other words, the absolute value of ΔGAl−induced for tetragonal phase HfO2 is larger than that for monoclinic phase HfO2. Therefore, the change of the Gibbs free energy of tetragonal phase for Al-doped HfO2 is larger than that for monoclinic phase and the energy difference between the two phases is reduced. Theoretical study by using the first-principles calculations of doped HfO2 has also indicated a similar conclusion.[27,28] Moreover, the decrease in the nucleation barrier for tetragonal phase
. After 550 °C annealing, Al atoms remain in Al2O3 layer because there is no enough thermal energy to break down Al–O bond, and the incorporation of Al2O3 can increase the thermal stability of HfO2 film as reported before. After 650 °C annealing, Al atoms will diffuse from Al2O3 layer into HfO2 layer and substitute Hf atoms thus causing additional strain energy ΔGAl−induced associated with the local lattice distortion. As the result of thermal fluctuation and by chance, a local atomic rearrangement will occur such that a new phase particle is created compatible to the thermodynamically preferred phase. The formation of new phase around Al atoms can gradually release part of the strain energy with the particle growing. It follows from Fig. 5(b) that ΔGAl−induced can result in a decrease in the nucleation barrier G* for either monoclinic or tetragonal phase HfO2. On the other hand, for alumina, the Al coordination number has a value between 4 and 6, and the Al–O bond lengths are between 1.71 Å and 1.79 Å.[25] For monoclinic phase HfO2, the Hf coordination number is seven and the average Hf−OI and Hf−OII bond lengths are 2.086 Å and 2.197 Å, respectively.[26] For tetragonal phase HfO2, the Hf coordination number is eight and the Hf–O bond lengths are equally distributed at 2.07 Å and 2.37 Å.[27] Obviously, there are four shorter Hf–O bonds in tetragonal phase HfO2 that will facilitate the formation of Al–O bonds with relatively small lattice distortion. In other words, the absolute value of ΔGAl−induced for tetragonal phase HfO2 is larger than that for monoclinic phase HfO2. Therefore, the change of the Gibbs free energy of tetragonal phase for Al-doped HfO2 is larger than that for monoclinic phase and the energy difference between the two phases is reduced. Theoretical study by using the first-principles calculations of doped HfO2 has also indicated a similar conclusion.[27,28] Moreover, the decrease in the nucleation barrier for tetragonal phase 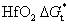 is larger than that for monoclinic phase
is larger than that for monoclinic phase 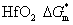 . Hence, 650-°C annealing can supply enough thermal agitation to rearrange the involved Hf atoms and O atoms to form a tetragonal phase nucleus. As can be seen in our GIXRD result, a multiphase consisting of monoclinic phase and tetragonal phase is formed in the Al-doped HfO2 film when being annealed at temperatures above 650 °C and the tetragonal phase is dominant. G0, ΔG, and ΔGʹ are expressed as
. Hence, 650-°C annealing can supply enough thermal agitation to rearrange the involved Hf atoms and O atoms to form a tetragonal phase nucleus. As can be seen in our GIXRD result, a multiphase consisting of monoclinic phase and tetragonal phase is formed in the Al-doped HfO2 film when being annealed at temperatures above 650 °C and the tetragonal phase is dominant. G0, ΔG, and ΔGʹ are expressed as
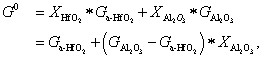 | (1) |
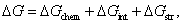 | (2) |
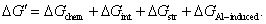 | (3) |