† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11474345, 11674043, and 11604030) and the Fundamental and Advanced Research Program of Chongqing (Grant No. cstc2018jcyjAX0338).
Development of an in vitro three-dimensional (3D) model that closely mimics actual environment of tissue has become extraordinarily important for anti-cancer study. In recent years, various 3D cell culture systems have been developed, with multicellular tumor spheroids being the most popular and effective model. In this work, we present a microfluidic device used as a robust platform for generating core–shell hydrogel microspheres with precisely controlled sizes and varied components of hydrogel matrix. To gain a better understanding of the governing mechanism of microsphere formation, computational models based on multiphase flow were developed to numerically model the droplet generation and velocity field evolution process with COMSOL Multiphysics software. Our modeling results show good agreement with experiments in size dependence on flow rate as well as effect of vortex flow on microsphere formation. With real-time tuning of the flow rates of aqueous phase and oil phase, tumor cells were encapsulated into the microspheres with controllable core–shell structure and different volume ratios of core (comprised of alginate, Matrigel, and/or Collagen) and shell (comprised of alginate). Viability of cells in four different hydrogel matrices were evaluated by standard acridine orange (AO) and propidium iodide (PI) staining. The proposed microfluidic system can play an important role in engineering the in vitro micro-environment of tumor spheroids to better mimic the actual in vivo 3D spatial structure of a tumor and perfect the 3D tumor models for more effective clinical therapies.
With the continuing increase in the rate of its incidence and mortality, cancer has become a pressing health issue around the world and has been receiving increased attention. Breast cancer is the most common type of cancer for women and is estimated to account for 15% of the confirmed cancer cases in 2015.[1] To develop effective cancer therapies, a suitable cell culture system has to be utilized. Studies have shown that almost all cells in vivo are exposed to a 3D environment containing various types of cells and complex extracellular matrix (ECM).[2,3] In traditional two-dimensional (2D) cell culture systems, cells can only attach to the flat solid surface of a petri dish, lacking cell–cell and cell–ECM interactions. Thus, the formation of 3D structure cannot be studied, not to mention the interaction between cancer cells and the 3D environment.[4,5] Moreover, it has been reported that in vitro cell cultures in 3D environment are advantageous over those in 2D environment for oncology studies, such as signal transduction, protein and gene expression.[2,6–9] Although a lot of efforts have been made during the past years, constructing an in vitro tumor model with complex ECM micro-environment remains a long-term challenge.
To overcome the disadvantages in 2D cell culture, an efficient model of 3D cell culture like multicellular tumor spheroids (MCTs) has been studied. MCTs can better mimic the actual 3D structural organization presented by solid tumors.[5,10] Several methods have been developed over the years to form MCTs, such as hanging drop,[11,12] spinner flasks,[13] ultra-low attachment plates,[14] and microfluidics.[15] Among these methods, microfluidic encapsulation of cells within hydrogel material has attracted more attention because it has unique advantages over traditional technologies including: (i) continuous, high-throughput and highly monodisperse production of 3D hydrogel-based cellular micro-environments with precisely controlled sizes, components and mechanical properties of hydrogel matrix;[16,17] and (ii) high porosity of hydrogel matrix, which guarantees good permeability of gas and liquid and provides a suitable growing condition for cell growth.[18]
To date, hydrogel scaffold materials of cell culture can be divided into two types: synthetic and natural. Alginate is a widely-used type of natural hydrogel with excellent biocompatibility and easy availability.[19–22] In previous studies, normally only one kind of hydrogel material was utilized to generate cell–laden hydrogel microspheres.[22,23] However, a basic in vitro 3D ECM model for reconstructing the actual environment of breast tumor should at least include both the basement membrane (Matrigel) and the interstitial matrix (Collagen).[24,25] Matrigel, mainly composed of proteins of ECM, optimizes spatial architecture of MCTs and facilitates cell function and behavior.[26,27] Collagen I is the major component of the naturally-derived matrix and plays an important role in cell proliferation and migration.[28,29] To create microspheres containing multiple hydrogel materials, microfluidic chips were utilized to fabricate emulsions with core–shell structure in a single step, allowing precise adjustment of the core size and shell thickness, as well as the heterogeneous micro-environment of core–shell droplets.[30] The heterogeneous structure provides a more efficient platform for oncology studies, such as cancer cells metastasis and heterotypic cell–cell interactions.
Core–shell hydrogel microspheres are designed to provide more effective in vitro tumor models, given that Matrigel and Collagen I are the major components of the ECM in breast tissue. In this paper, we present a droplet-based microfluidic device for generating heterogeneous core–shell microspheres which incorporate ECM components including both Matrigel and collagen I. The microfluidic device can be used as a robust platform for generating core–shell hydrogel microspheres with precisely controlled sizes and varied components of hydrogel. The dynamics of droplet generation and velocity field evolution in the microfluidic device were investigated by both experiments and numerical simulation. The simulation results demonstrate good agreement with experimental observations. We can adjust the composition of core and shell in real-time by adjusting the flow rate of the inner and outer aqueous phase. the variability of the compositions of core and shell makes it a robust platform for real-time study. In the core, 3D hydrogel environment with the addition of ECM components including both collagen I and reconstituted basement membrane provides a good growth micro-environment for cells compared with those containing only one kind of hydrogel material. Cell growth in different hydrogel components was also evaluated in detail.
The phase field method is adopted to solve the evolution of interface between immiscible aqueous and oil phase. The interfacial layer between aqueous and oil phase is governed by a phase field variable ϕ, which takes a value from –1 (pure aqueous phase) to 1 (pure oil phase). The velocity and pressure field is governed by the Navier–Stokes equation:
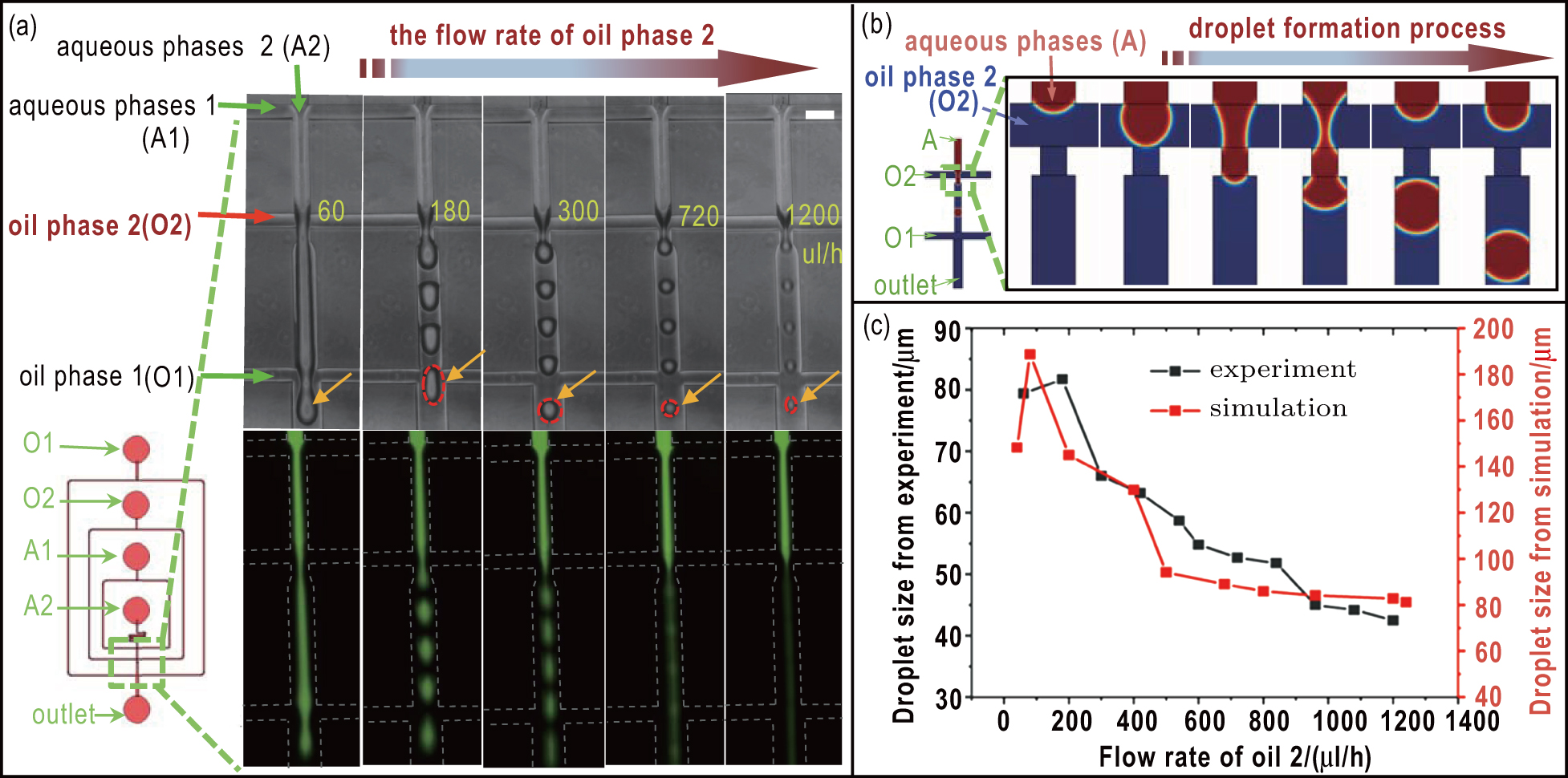
The Two-Phase flow, phase field module in COMSOL Multiphysics 5.3a was employed to numerically model the droplet generation and analyze the change of flow field in 2D geometry. The geometry used in the computational work was the same as that used in the experiments. Sodium alginate solution was chosen as the aqueous phase, and fluorinated carbon oil supplemented with 1-wt% biocompatible surfactant was used as the oil phase. The aqueous phase has a viscosity of 8 mPa·s and a density of 1250 kg/m3 while the oil phase has a viscosity of 1.24 mPa.s and a density of 1614 kg/m3. Surface tension values of 0.037 N/m and 0.0162 N/m were assigned to the aqueous and oil phase, respectively.
For the boundary conditions, the velocity of aqueous phase and phase field variable ϕ = –1 were assigned to the top inlet A as shown in Fig.
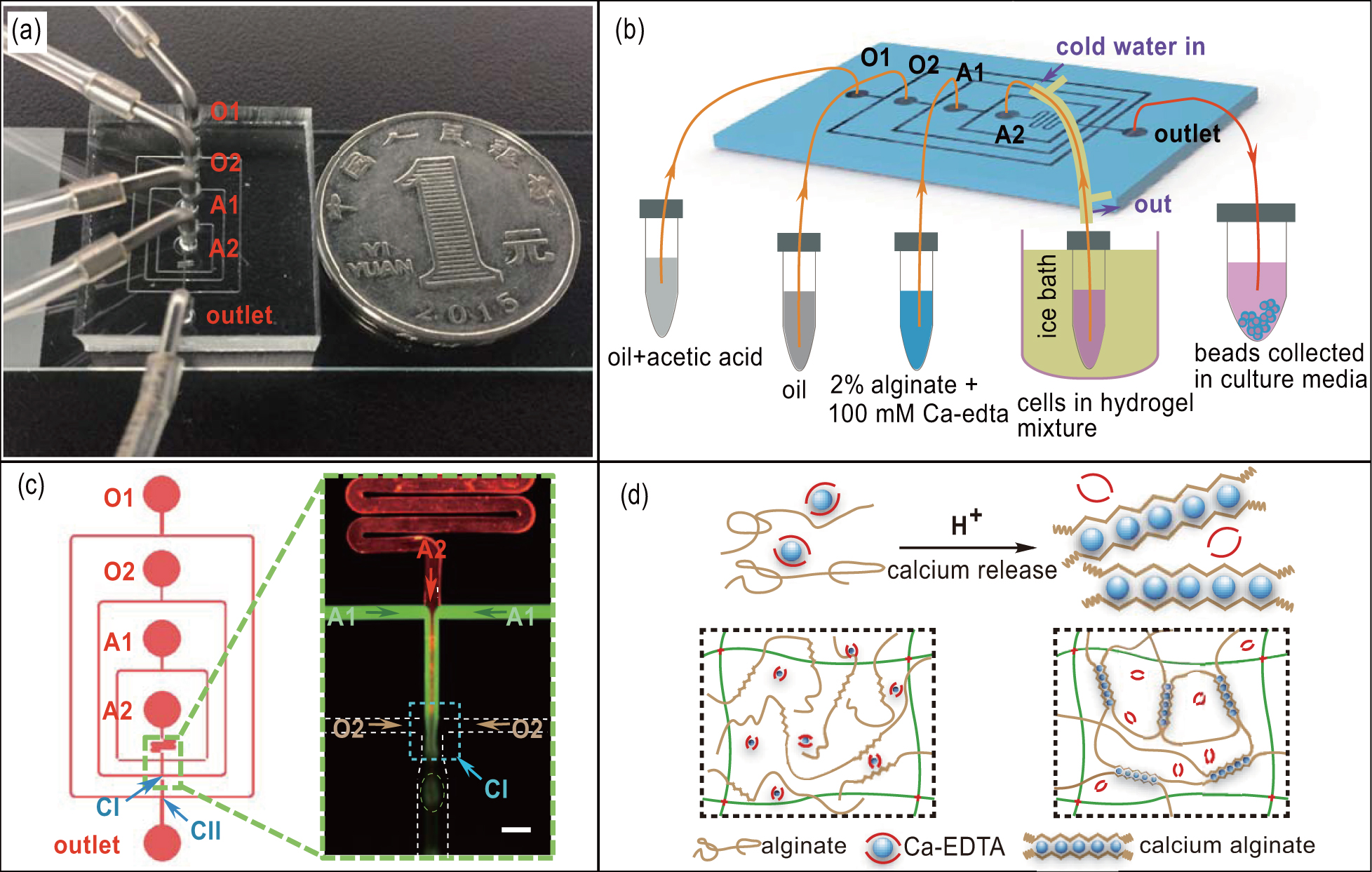
We used soft lithographic technique[31,32] to fabricate the PDMS microfluidic chip for droplets formation. Briefly, the pattern of PDMS chip was designed with the L-edit software (Tanner EDA). The designs were then printed onto a 5-inch (1 in = 2.54 cm) chrome mask using a laser writer (Heidelberg DWL2000 Mask Writer). Silicon wafers were carefully cleaned and spin-coated with positive photoresist (ECL3027). The photoresist pattern was created by standard UV lithography techniques. After development, the silicon was treated by O2 plasma to remove the residual photoresist outside the microchannel pattern. Then the exposed silicon regions were etched down to 150 μm. The fabricated silicon wafer was transferred to a plastic 100-mm diameter petri dish to be used as the master mold. Poly(dimethyl siloxane) (PDMS, Dow Corning) was first mixed well with curing agent (Dow Corning) in 10:1 (w/w). The PDMS mixture was then poured onto the master mold to degas in a vacuum desiccator for 15 minutes and cured as PDMS in 60 °C oven for 2 h. Next, a commercially available 2-mm diameter punch (Harris Uni-Core) was utilized to punch the through-holes at each channel inlet and outlet. Subsequently, the PDMS replicas of the desired pattern were bonded to glass slides after oxygen plasma treatment. To avoid wetting of the channel of microfluidic chip by water phase and ensure stabilized droplet production, all of the fabricated channels in microfluidic chip were treated with commercial water repellent Aquapel (PPG Industries, USA).[33]
The sodium alginate powder (Sigma–Aldrich) was dissolved in cell culture medium to a final concentration of 2% (w/v). The calcium-EDTA solution (100 mM) was prepared by using a solution of calcium chloride (Sigma–Aldrich) mixed with disodium-EDTA (Sigma–Aldrich) at a ratio of 1:1 (v/v). Subsequently, the pH value of the calcium-EDTA solution was adjusted to 7.2 using 1-mol/L NaOH (Fluka).
The alginate mixture for droplet generation was obtained by mixing 2% sodium alginate with 100-mM calcium-EDTA at the volume proportion of 1:1, and then vortexed to ensure thorough mixing of the two solutions.[22] To prevent clogging in microfluidic chips and sterilize the solution, alginate mixture was filtered using 0.2-μm syringe filters (Millipore) to remove any clumps of alginate or bacteria.[34] The alginate/calcium-EDTA solution was utilized as the aqueous phase for droplet generation.
The oil phase used in our study was fluorinated carbon oil (HFE7500, 3 M) supplemented with 1-wt% biocompatible surfactant (RainDance Technologies) and with or without 0.15% (v/v) acetic acid.[22] To stabilize the monodispersed droplets, the surfactant was used to prevent uncontrolled droplet coalescence prior to gelation. The acetic acid can reduce the pH level of dispersed aqueous phase, and eventually lead to alginate gelation.
The microfluidic chip with a flow-focusing geometry was utilized in our study to generate highly monodisperse alginate droplets.[35,36] By using a long tube with 0.8-mm inner diameter and 2.4-mm outer diameter, the inlets of microfluidic chip were connected to plastic syringes (BD Falcon) with 19-gauge flat tip needles, and the outlet was also routed into a plastic centrifuge tube (Corning) for droplets collection as shown in Fig.
Human breast carcinoma cell line MDA-MB-231 (China Infrastructure of Cell Line Resource, Beijing, China) was cultured in DMEM (Corning) supplemented with 10% Fetal Bovine Serum (Corning) and 1% penicillin/streptomycin (Corning). Before loaded into the microfluidic chip, MDA-MB-231 cells were cultured in flasks to 80% confluence and rinsed twice with phosphate buffered saline (Corning) and then detached using 0.25% trypsin-EDTA (Corning) solution. Cell suspension was prepared with a concentration of 1×107 cells/mL using cell culture medium mixed with ECM-based hydrogel solution on ice. Hydrogel droplets were generated as described in results and discussion section. After droplets gelation, the gelled hydrogel microspheres were collected using a 40-μm cell strainer (BD Falcon), and then rinsed with 1% (w/v) CaCl2 once to enhance mechanical properties and PBS twice to discard the remaining oil phase solution, followed by re-dispersing in 24-well plate (Corning) with adequate cell culture medium. The cell–laden hydrogel microspheres were cultured in an incubator with 5% CO2 at 37 °C and the culture medium was changed every two days during the experiments.
Cell viability was assessed using a standard acridine orange (AO) and propidium iodide (PI) staining protocol.[37] Briefly, after removing the culture medium and rinsing samples in 1 ml of PBS for 5 min at 37 °C, the cell–laden hydrogel was incubated in 5-μg/mL AO staining solution to stain living cells and 5-μg/mL PI to stain dead cells for 15 min at room temperature. After incubation, the hydrogel was then rinsed in PBS and imaged under an inverted microscope (Ti, Nikon) with a 10/20x air objective in 25-°C environment. The images and data have been processed and quantitatively analyzed later using the ImageJ and Origin software package, respectively.
We aimed to fabricate a simple and stable microfluidic device which can generate highly monodisperse alginate droplets and encapsulate tumor cells over a long period of time. As shown in Figs.
The microfluidic chip that we used in our study can generate droplets with stable and uniform size. The process of droplet generation is affected by a group of independent variables, including flow rates, viscosity of medium and the geometry and dimension of the microchannel. We first investigated the effect of flow rate of the oil phase on the size of droplets. Figure
It is easy for the quickly ionically cross-linked alginate hydrogels to produce hydrogel microspheres with uncontrollable size and shape, which can clog the microchannel within several seconds. To solve this problem, we used water-soluble calcium-EDTA mixed with alginate as the aqueous phase in this study.[39] The reaction scheme is shown in Fig.
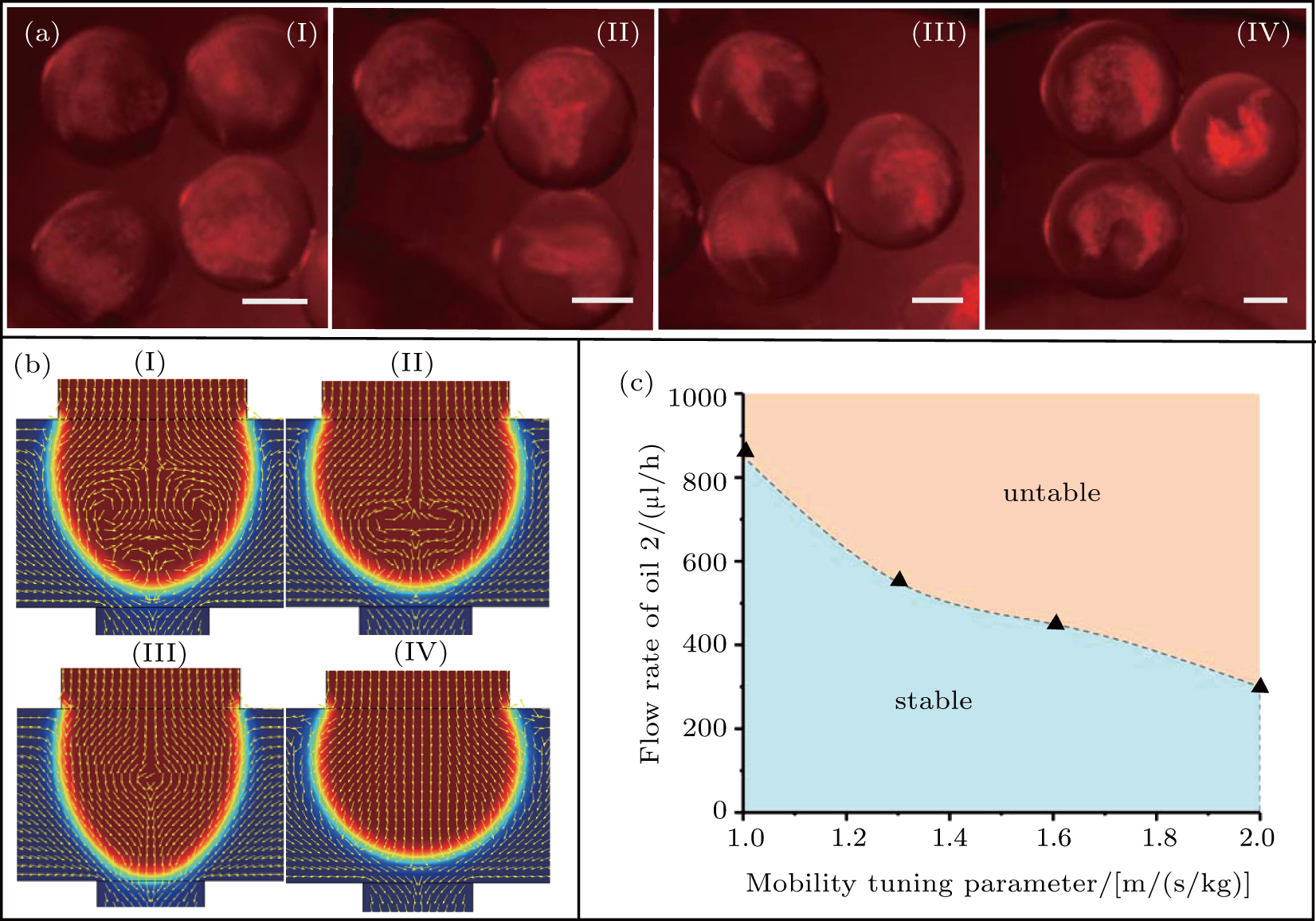
To generate core–shell hydrogel microspheres, the Matrigel and Collagen mixture with or without tumor cells was used as the aqueous 2 (A2), and the alginate/calcium-EDTA mixture was used as the aqueous 1 (A1). Fluorinated carbon oil supplemented with 0.15% (v/v) acetic acid and without acid were used as oil 1 (O1) and oil 2 (O2), respectively. The flow rate of the aqueous phase for all cases were 120 μL/h. Figure
The value of mobility tuning parameter χ has an effect on the critical flow rate of oil 2 at which recirculation is initiated. Figure
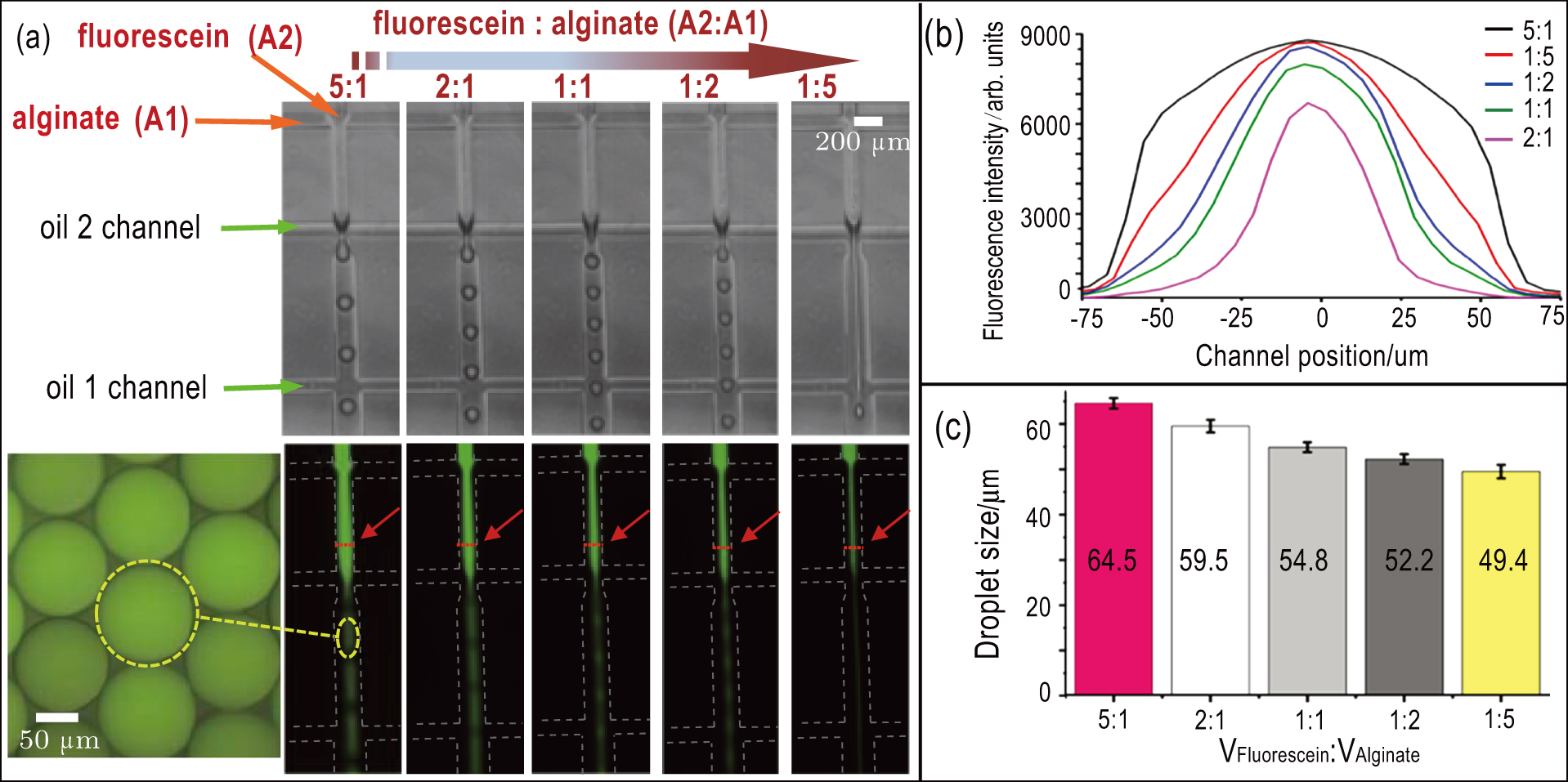
The heterogeneous core–shell structure of cell–laden hydrogel microspheres provide a more complex and realistic 3D micro-environment for mimicking the in vivo 3D spatial structure of tumor. The microfluidic chip can generate hydrogel droplets with variable volume ratios of core to shell and adjust the ratio in real-time (Fig.
To generate cell–laden core–shell hydrogel microspheres, all of the fabricated PDMS microfluidic chips were sterilized with 75% ethanol and UV light irradiation for 30 minutes before cell encapsulation. By changing the flow rate of the inner and outer aqueous phases, we can adjust the composition of droplets. Figures
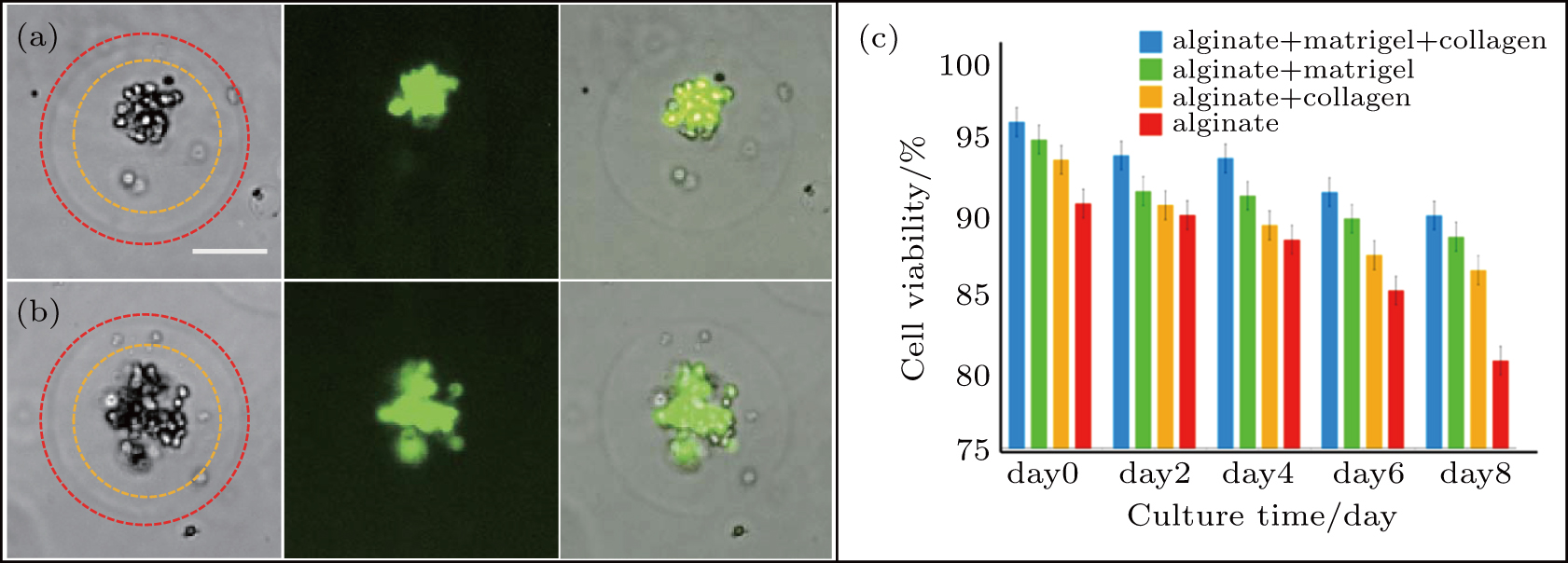
The viability of encapsulated cells in four different hydrogel matrices were assessed at 0, 2, 4, 6, and 8 days.[37] Initially, cells are sparsely dispersed in the hydrogel matrix. After 2 hours culture, assay results show more than 92% viability as shown in Fig.
A microfluidic device has been developed to generate heterogeneous core–shell microspheres that incorporate ECM components including both Matrigel and collagen I. The dynamics of droplet generation and velocity field evolution in the microfluidic device were investigated by both experiments and numerical simulation. The simulation results demonstrate good agreement with experimental observations. The microfluidic device provides a more efficient platform to manipulate cells and their environment, especially for oncology studies, such as anticancer drugs, cancer cells metastasis and tissue model studies, as the heterogeneous core–shell microspheres potentially provide well mimicked ECM micro-environment to observe cell–cell and cell–ECM interactions. Furthermore, the variability of the compositions of core and shell makes it a robust platform for real-time study, such as proliferation within natural ECM materials or invasion into hydrogel matrix under an engineered 3D micro-environment.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] |