† Corresponding author. E-mail:
In this manuscript, the perovskite-based metal–oxide–semiconductor field effect transistors (MOSFETs) with phenyl-C61-butyric acid methylester (PCBM) layers are studied. The MOSFETs are fabricated on perovskites, and characterized by photoluminescence spectra (PL), x-ray diffraction (XRD), and x-ray photoelectron spectroscopy (XPS). With PCBM layers, the current–voltage hysteresis phenomenon is effetely inhibited, and both the transfer and output current values increase. The band energy diagrams are proposed, which indicate that the electrons are transferred into the PCBM layer, resulting in the increase of photocurrent. The electron mobility and hole mobility are extracted from the transfer curves, which are about one order of magnitude as large as those of PCBM deposited, which is the reason why the electrons are transferred into the PCBM layer and the holes are still in the perovskites, and the effects of ionized impurity scattering on carrier transport become smaller.
As a potential photoelectric material, organic–inorganic halide perovskite (OIHP) has drawn tremendous attention in the past several years.[1–5] Perovskites in the form of ABX3 (A = methylammonium, B = lead, X = chlorine, bromine, and iodine) exhibit excellent properties such as long electron diffusion lengths, the ability to strongly absorb light, and extremely low preparation cost.[6–8] The power conversion efficiency of the OIHP based solar cells has increased from 3.8% in 2009 to 21% in 2016.[9–12] Perovskite based photovoltaic devices, such as photodetectors, lasers, and phototransistors have been widely analyzed in recent years.[13–18] However, OIHPs are not applied to industrial production since the film can be easily decomposed in the air, and the mobile ions like iodine− and methylammonium+ will affect the stability and reliability of the photovoltaic device.[12,19,20]
The study of the ion migration in the OIHP film focused on how the ions move and how the ions inhibit.[21,22] Yang et al.[20] and Xu et al.[23] performed the density functional theory calculations of both the diffusion barriers and the formation energies for MA+, I−, and Pb2+. They found that the I− and MA+ have lower diffusion barriers than the Pb2+.[20,23] The phenyl-C61-butyric acid methylester (PCBM) was often used for the inhibition of the iodine ions.[24] Liu et al. proposed a new model based on the hindering theory of ions (vacancies) migration with PCBM.[19] Xu et al. proved that the PCBM phase promotes electron extraction and passivates the key 
In the present study, the structures (#1: perovskite, #2: PCBM/perovskite/PCBM) of OIHP-based phototransistors are prepared and discussed. The perovskite films are characterized by photoluminescence spectra (PL), x-ray diffraction (XRD) and x-ray photoelectron spectroscopy (XPS). Current–voltage (I–V) curves are measured and the channel carrier mobility is calculated. The effects of the PCBM layers on the perovskite MOS field-effect transistors are presented by energy band and transport loops diagrams.
The 400-

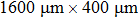

 | Fig. 1. (color online) Structures for MOSFETs (#1) Au/perovskite/SiO2/Si/Al and (#2) Au/PCBM/perovskite/PCBM/SiO2/Si/Al. |
The XRD, XPS, and PL measurements are presented in Fig.
The transfer characteristic curves of the transistors, measured in the dark, are shown in Figs.
 | Fig. 3. Drain current–gate voltage curves at Vd = -5 V in ((a) and (b)) the dark and ((c) and (d)) light condition. |
The output current curves in the dark and under illumination (2 mW/cm2) are illustrated in Figs.
 |





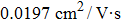


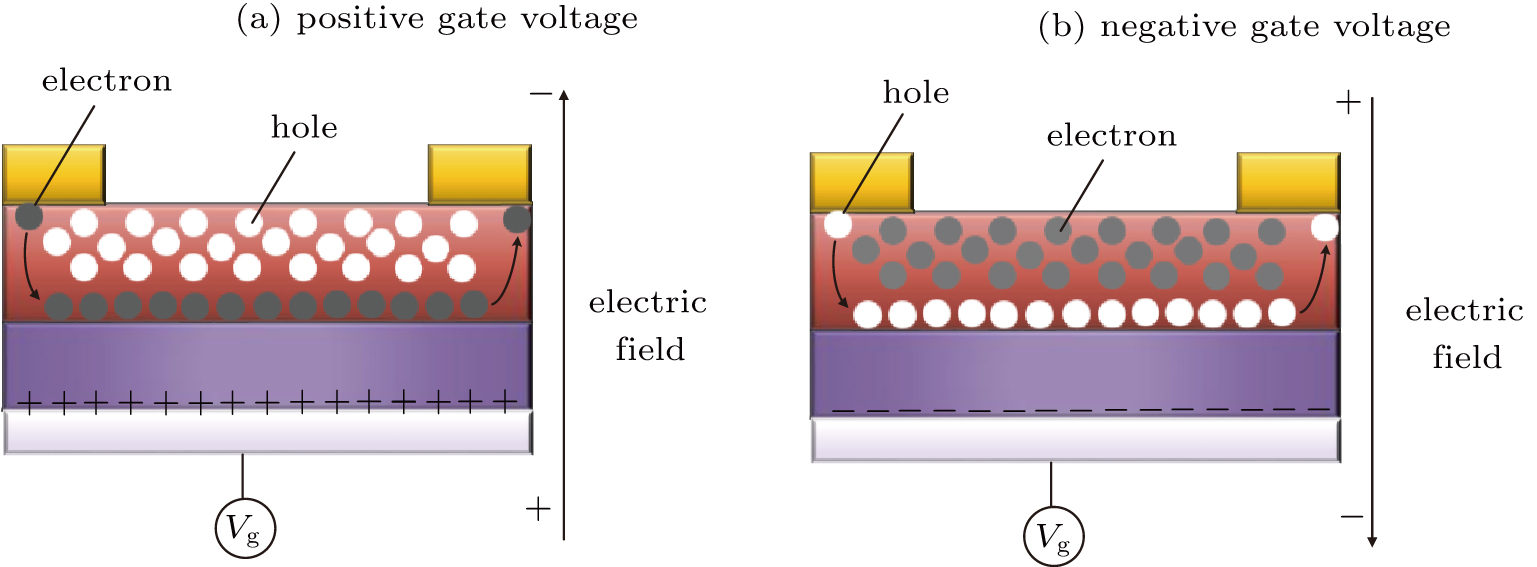
In this study, the effects of perovskite based MOSFETs with and without PCBM are discussed. With PCBM layers, the current–voltage hysteresis phenomenon is effetely reduced, and both the transfer and output current values increase. The band energy diagrams are proposed, which indicate that the electrons are transferred into the PCBM layer, resulting in the increase of photocurrent. By fitting the MOSFETs transfer curves, the values of electron and hole gate mobility under light condition are calculated. With PCBM layers deposited in MOSFET, the electrons are transferred into the PCBM layer and the holes are still in the perovskites, and the effects of ionized impurity scattering on carrier transport become smaller. The electron mobility and hole mobility are, respectively, 

| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 |