† Corresponding author. E-mail:
Project supported by the National Key Research and Development Program of China (Grant Nos. 2016YFA0401503 and 2018YFA0305700), the National Natural Science Foundation of China (Grant No. 11575288), the Youth Innovation Promotion Association of Chinese Academy of Sciences (Grant No. 2016006). the Key Research Platforms and Research Projects of Universities in Guangdong Province, China (Grant No. 2018KZDXM062), the Guangdong Innovative & Entrepreneurial Research Team Program, China (Grant No. 2016ZT06C279), the Shenzhen Peacock Plan, China (Grant No. KQTD2016053019134356), the Shenzhen Development & Reform Commission Foundation for Novel Nano-Material Sciences, China, the Research Platform for Crystal Growth & Thin-Film Preparation at SUST, China, and the Shenzhen Development and Reform Commission Foundation for Shenzhen Engineering Research Center for Frontier Materials Synthesis at High Pressure, China.
As one of important members of refractory materials, tungsten phosphide (WP) holds great potential for fundamental study and industrial applications in many fields of science and technology, due to its excellent properties such as superconductivity and as-predicted topological band structure. However, synthesis of high-quality WP crystals is still a challenge by using tradition synthetic methods, because the synthesis temperature for growing its large crystals is very stringently required to be as high as 3000 °C, which is far beyond the temperature capability of most laboratory-based devices for crystal growth. In addition, high temperature often induces the decomposition of metal phosphides, leading to off-stoichiometric samples based on which the materials’ intrinsic properties cannot be explored. In this work, we report a high-pressure synthesis of single-crystal WP through a direct crystallization from cooling the congruent W–P melts at 5 GPa and ∼ 3200 °C. In combination of x-ray diffraction, electron microscope, and thermal analysis, the crystal structure, morphology, and stability of recovered sample are well investigated. The final product is phase-pure and nearly stoichiometric WP in a single-crystal form with a large grain size, in excess of one millimeter, thus making it feasible to implement most experimental measurements, especially, for the case where a large crystal is required. Success in synthesis of high-quality WP crystals at high pressure can offer great opportunities for determining their intrinsic properties and also making more efforts to study the family of transition-metal phosphides.
Transition-metal (TM) phosphides have been widely studied in the fields of industrial catalysis,[1–11] magnetic refrigeration systems,[12–14] superconductivity,[15–19] optoelectronic devices, and semiconductors[20,21] due to their exceptional structural, electronic, magnetic, and catalytic properties. For most of binary TM phosphides, there often exist a large number of compounds with different compositions, mainly because the transition metals possess many d electrons, thus making it allowable that their oxidation states can be largely varied. In the W–P binary system, a series of materials has been reported including tetragonal W3P[17] and WP4,[22] orthorhombic WP,[2] and WP2,[23] etc. Over the past decades, they have been intensively studied mainly due to their intriguing electronic properties for the study of diverse fundamental sciences, such as superconductivity (e.g., W3P),[17] Weyl semimetals (e.g., WP2).[24,25] Among them, WP is a shining member of phosphides, exhibiting superconductivity of a weak-coupling BCS type.[18] Very recently, based on the first-principles calculations, it has been predicted that WP possesses a unique topological band structure.[26] But such a theoretical prediction has not yet been validated experimentally, because the preparation of high-quality single-crystal WP samples is still a challenge by traditional reaction routes at atmospheric pressure. As a matter of fact, a similar issue also occurs for most of TM phosphides, which severely restricts the scientific study on this whole class of materials.
Traditional methods for synthesizing WP include the element reaction by ampoule techniques and arc melting,[2] the reduction of phosphate precursors in hydrogen,[6] the hydrogen plasma reduction,[7] and the decomposition of organometallics.[2] However, most of the obtained samples are poorly-crystallized and in nanocrystalline forms, which have been employed as catalysts for hydrogenating process.[2–5] Obviously, those methods cannot be used for preparing the high-quality WP crystals, because of the limited thermal stability of this compound at ambient pressure with a low decomposition temperature of ∼ 1000 °C, even lower than its synthesis temperatures. As a result, the composition of final products often deviates from its ideal stoichiometry. The thus-degassed phosphor gas for traditional methods is chemically active and toxic, which may contaminate and damage the expensive furnace devices. Growing large single crystals of materials from their high-temperature melts is one of mostly exploited approaches, but it is almost impossible for refractory materials with very high melting points. Taking WC for example, its ambient-pressure melting point is close to ∼ 3000 °C,[27] which is far beyond the temperature capability of most laboratory-housed furnace devices. As a sister material, the WP should also have an extremely high melting point. As such, disadvantages of traditional synthetic methods have posted a great challenge to the preparation of high-quality WP single crystals, although chemical vapor transport (CVT) technique has been used for growing single-crystal WP[18] or WP2,[24] it still has some problems to be solved, such as high experimental cost, slow synthesis rate, low quality, and small-scale production.
High pressure (P) and temperature (T) synthesis has proven to be an effective route for the synthesis of TM-bearing compounds or for the discovery of their novel materials including borides, nitrides, and phosphides, because the oxidation states of metals tend to vary with pressure. Accordingly, a number of novel nitrogen-rich TM nitrides have recently been discovered under pressures in the Zr–N,[28,29] Hf–N,[28] Ta–N,[30,31] Mo–N,[32] W–N,[33] and noble metal nitride systems,[34–37] suggesting that the pressure can effectively suppress the high-T degassing and promote the involvement of d-electrons in chemical bonding.[38] Successful syntheses have also been achieved in the TM phosphides of ZnP4,[39] Ir2P,[40] and Cd–P (e.g., CdP4, and Cd7P10)[41,42] and borides of HfB2[43] and TiB2.[44] Based on large-volume high-P devices, the millimeter-sized GaN single crystals have recently been grown from its continently melted liquid at 6 GPa and 2200 °C;[38] conversely, the complete decomposition of GaN has also been observed below 6 GPa, further confirming the crucial role of pressure played in this process. However, for materials with very high melting points (i.e., above 3000 °C), it is difficult to implement this method for growing their single crystals, because for most available large-volume apparatus their temperature conditions are limited to ∼ 2300 °C. Very recently, we have re-designed a new high-P cell assembly of large-volume cubic press and significantly optimized its heating efficiency, leading to extraordinarily extended P–T conditions of 10 GPa and 3700 °C.[27] Using the optimized cell, large single-crystal refractory materials of Mo, Ta, and WC have been made,[27] indicating that a similar method can also be used for the growth of WP single crystals from its congruent melts.
With these aims, in this work we perform high P–T experiments for growing large single-crystal WP from congruently pre-melted W and P liquids at 5 GPa and temperature up to 3200 °C, leading the high-quality WP crystals with large crystallite size of a few millimeters to success in being prepared.
The purchased high-purity W powders and red phosphorus were used as starting materials for high P–T growth of crystals. Prior to the experiment, W and P powers were homogenously ground and mixed in a molar ratio of 1 : 1. The mixed powders were compacted into a cylinder with 8 mm in diameter and 4 mm in height, which was surrounded by hBN capsule. Note that the hBN could dissolve into the W–P melts at high temperature; during cooling, it precipitated on the surface of each crystal, which facilitated the separation of crystals to avoid the formation of polycrystalline bulk continuum. High-P experiment was carried out in a 6 × 10 MN large-volume cubic press installed at the high-P laboratory of SUSTech. At the target pressure, the sample was gradually heated to the desired temperature. For a safe operation of pressure, the heating process was carefully program-controlled. Depending on the required experiment temperature, the heating duration was strictly restricted, in avoidance of blowout. Accordingly, at temperatures of 1800 °C, 2400 °C, 2700 °C, 3000 °C, and 3200 °C, the holding time for heating were 30 min, 5 min, 2 min, 1 min, and 0.5 min, respectively, followed by cooling with a rate of 300 °C/min. More experimental details can be found elsewhere.[27,45,46] The recovered samples were characterized by x-ray diffraction (XRD), scanning electron microscope (SEM), and thermogravimetric-differential thermal analyzer (TG–DTA). Refinement of crystal structure was performed by using the Rietveld method and the FullProf program.[47]
In addition, single-crystal diffraction was also performed to check the quality of as-prepared crystals by using the single crystal x-ray diffractometer (BRUKER D8 VENTURE) and Laue diffractometers. Energy dispersive spectrometer (EDS) and electron probe micro-analyzer (EPMA) measurements were conducted to analyze the chemical composition of samples.
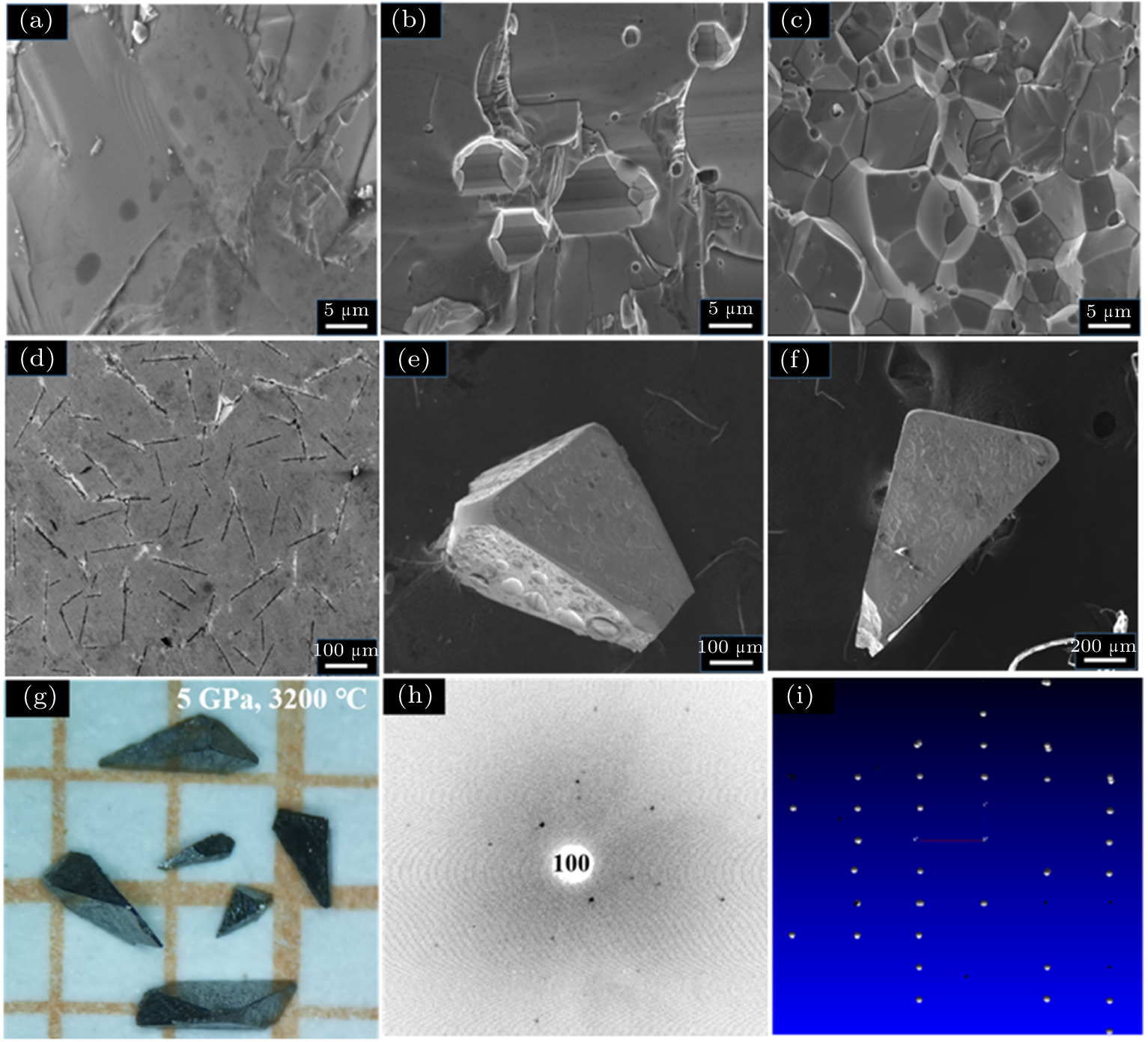
In order to understand the high P–T reaction process for the formation of WP from reaction between W and P, a number of synthesis experiments are performed at pressures of 1.0 GPa–6.0 GPa and temperatures of 400 °C–3200 °C. Figure
Figure
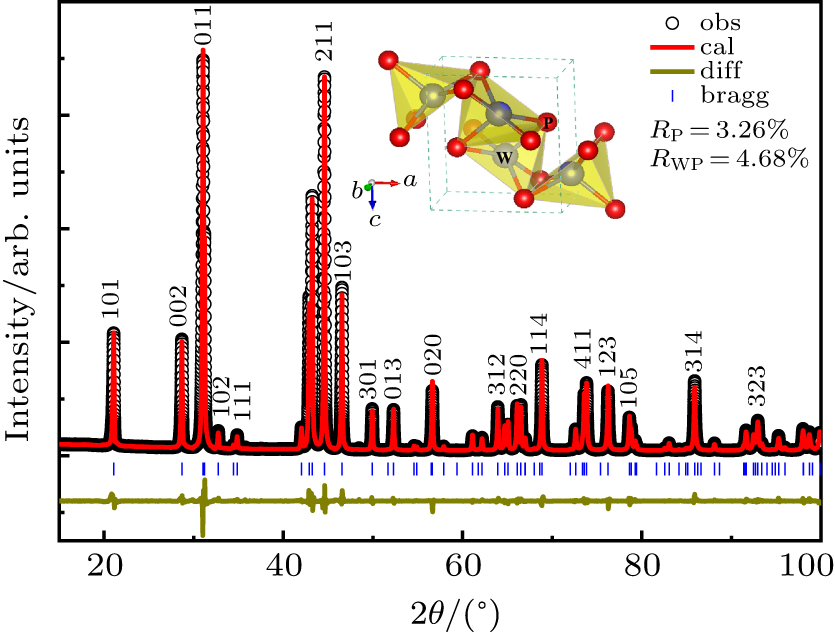 | Fig. 2. Refined XRD pattern of polycrystalline WP synthesized at 5 GPa and 1600 °C taken at ambient conditions by using a copper radiation. Inset shows a polyhedral view of its crystal structure. |
As presented in Figs.
To our surprise, large WP crystals occur as temperature exceeds 3000 °C, the results are shown in Figs.
To further check the quality of such-prepared WP crystals, we conduct single-crystal diffraction measurements, and the results are shown in Figs.
| Table 1. Refined crystal structure parameters for WP by using single-crystal diffraction data. . |
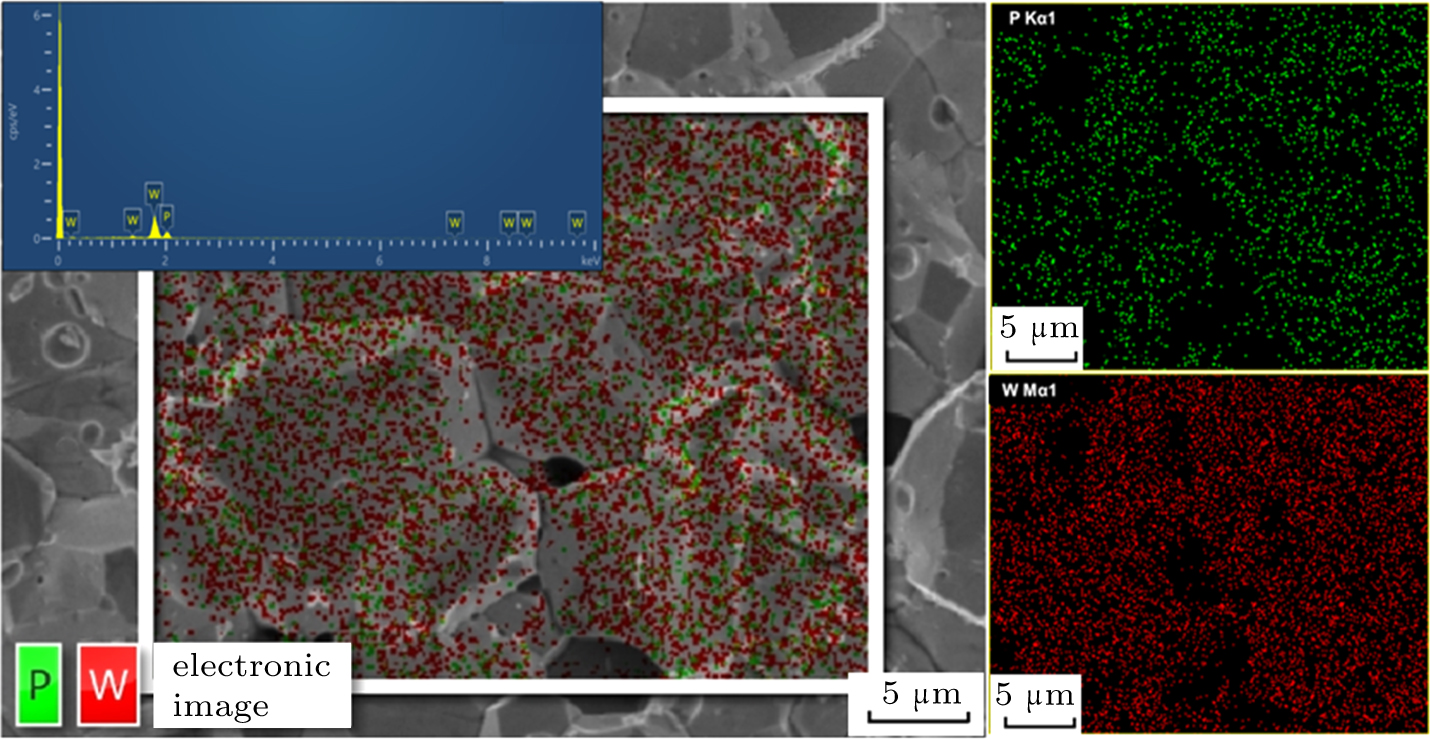
To determine the chemical composition of WP crystals, we also perform EDS and EPMA measurements on high-P synthesized WP crystals, and the results are shown in Fig.
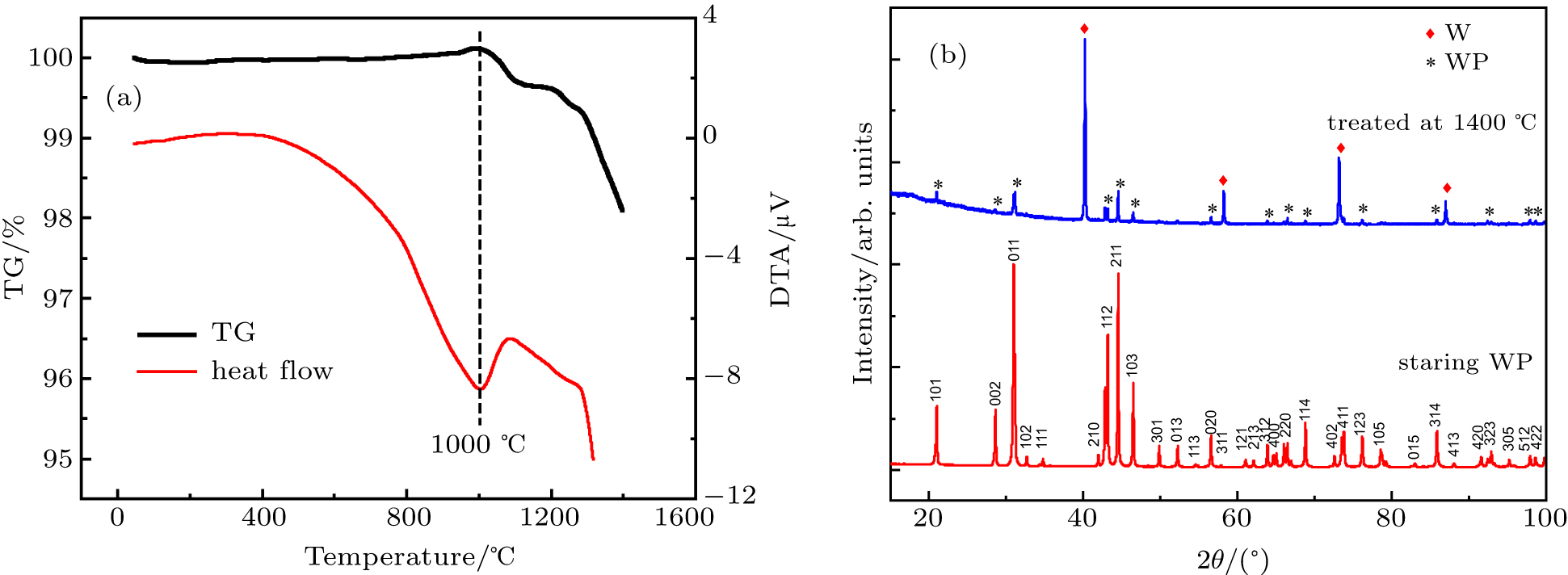
Finally, the thermal stability of WP is evaluated by using a regular TG-DAT analysis. As seen in Fig.
In this work, using our recently designed high-P cell, we successfully prepare large single-crystal WP from cooling the congruent melts of W–P at 5 GPa and 3000 °C–3200 °C. The obtained crystal possesses high quality with a crystallite size over one millimeter, which will facilitate further experimental determination of its intrinsic properties for further exploring the predicted interesting physics in this material such as topological band structure. Besides, the formulated synthetic methodology can also be exploited for growing high-quality crystals for most TM phosphides, which would open a new avenue to the study of condensed-matter physics and material science.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] |