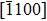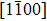† Corresponding author. E-mail:
As a representative of small aromatic molecules, triphenylene (TP) has markedly high carrier mobility and is an ideal precursor for building graphene nanostructures. We mainly investigated the adsorption behavior of TP molecules on Ru(0001) by using scanning tunneling microscopy (STM). In submonolayer regime, TP molecules are randomly dispersed on Ru(0001) and the TP overlayer can be thoroughly dehydrogenated and converted into graphene islands at 700 K. Due to weak interaction between TP molecules and graphene, the grooves formed among graphene islands have confinement effect on TP molecules. TP adopts a flat-lying adsorption mode and has two adsorption configurations with the 3-fold molecular axis aligned almost parallel or antiparallel to the 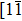
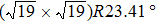
The rapid development of molecular electronics took off in tandem with the rise of the nanoscience era in the past decades.[1–4] Bottom-up self-assembly of molecular building blocks at interfaces has been an extremely powerful method for fabricating highly functional nanostructures and nanoscale electronic devices with tailored properties.[5–9] The versatility of organic molecular materials can be preserved by some symmetry requirements in molecular assemblies. Microscopic ordering significantly enhances the high performance of organic electronic and optoelectronic devices, such as organic field-effect transistors (OFETs), organic light-emitting diodes (OLEDs), or photovoltaic solar cells.[10] To date, molecular assemblies on novel two-dimensional (2D) materials,[11,12] topological insulators,[13] semiconductors,[14] and metals[15–18] have sprung up. However, molecular assemblies on surfaces are not necessarily well-ordered. Manganese phthalocyanine on Bi2Te3 shows poor ordering, cytosine on Au(111) exhibits a random pore network, and the second layer of diF-TES-ADA is disordered despite ordering in the first layer.[6] Disorder at or near metal–organic interfaces can also play an essential role in raising the efficiency of charge injection.[19] So it is fundamentally important to have a comprehensive understanding of adsorption behaviors of molecules at interfaces and further improve the organic/substrate device performance. Generally, molecular self-assembly on the solid surface is governed by the delicate balance of intermolecular and molecule–substrate interactions. The driving mechanism of molecular self-assembly largely comes from different non-covalent intermolecular forces, such as hydrogen bonding,[5,20] π–π stacking,[21,22] dipolar,[23] electrostatic,[24] van der Waals,[25] and metal–ligand[26] interactions. Nevertheless, it is still a big challenge to precisely control and engineer desired architectures in reality, which stems from the complex interactions between adsorbates and substrates, coupling with the molecular geometry and intricate molecular deposition processes.
Polycyclic aromatic hydrocarbons with unique electronic and optoelectronic properties have aroused widespread interest. Functionalization of surfaces modified by aromatic molecules which have very high carrier mobility on single crystals is the paramount approach towards novel materials. Triphenylene (TP) is among aromatic molecules with π-conjugated system and can behave as both electron acceptors[27] and donors.[28] For example, TP has a complementary electronic density with trinitrotoluene (TNT) in electronic characteristics,[29] forming a donor–acceptor complex, which facilitates the molecular recognition of TP to TNT.[30] Because of the extended π-system of TP, it can be a precursor to fabricate graphene-like materials, such as graphene nanoribbons or nanographenes. In addition, TP can act as the scaffold for building new molecular materials to form self-assembly patterns[31,32] and liquid crystals.[33–35] However, studies on TP adsorbed on metal substrates have rarely been reported.
The ordering of the molecular orientation is affected by various factors. The key to steering adsorption configurations is the control over the strength of the molecule–metal interaction and its spatial variation. Great efforts have been devoted to investigations of molecules assembling into diverse superstructures by varying the substrates, where different electronic structures of substrates may lead to a series of rich and fascinating phenomena. For instance, anthraquinone (AQ) molecules on Au(111)[6] form a disordered pore network in contrast with a highly ordered regular giant honeycomb pore network observed on Cu(111).[36] Ru is a prototype transition metal with unoccupied d-shell, which has stronger surface reactivity than noble metals, such as Au(111), Ag(111), and Cu(111). Epitaxial graphene on Ru(0001) presents a molecular adsorption energy landscape with substantial periodic variation due to strong interaction of π–d electrons. So high surface activity aids in capturing small molecules from desorption and promotes the conversion into graphene at lower temperature.
The organic molecules with several aromatic groups have been in the spotlight in molecular self-assembly due to their ubiquitous π interactions making a strong contribution to their structure and stability. The structures and morphologies of the molecules on different substrates determine the charge injection and charge transport. To better understand the microscopic underpinnings of such molecules adsorbed on active metals and achieve efficient charge transport at interfaces, we here investigated the adsorption behavior of TP on Ru(0001) by means of scanning tunneling microscopy (STM) at room temperature. We firstly present the conversion stage of TP molecules into graphene nanostructures at low coverage and then analyze the adsorption configurations of TP on Ru(0001) at different coverages. Finally, TP molecular self-assembly superstructures are exhaustively discussed.
The experiments were performed in a multi-functional ultrahigh-vacuum (HUV) VT-SPM system (Omicron Instruments for Surface Science) with a base pressure better than 2×10−10 mbar. The system was described in detail elsewhere.[37] In brief, it consists of a fast entry, a preparation chamber, an analysis chamber, and a scanning tunneling microscopy (STM) chamber. The system is equipped with an electron beam heating setup, a low-energy electron diffraction (LEED) attachment, an x-ray photoemission spectrometer, etc.
The Ru(0001) substrate was in situ cleaned by several cycles of argon ion sputtering (1500 eV×1 h) and subsequent annealing at 1273 K. The substrate surface then was confirmed to be atomically clean and flat by LEED and STM measurements before molecular deposition. The commercial triphenylene powder (98% purity) purchased from J&K Scientific Company was loaded in a homemade Ta boat and thoroughly degassed overnight in the fast-entry chamber. The Ta boat was also used to sublimate TP molecules onto the Ru(0001) substrate by direct current heating while keeping the substrate at room temperature. The deposition dosage was carefully controlled by the deposition rate and time. All the STM measurements were conducted in constant current mode using an electrochemically etched tungsten tip at room temperature and the given bias voltages were applied to the sample with respect to the tip. The experimental STM images were processed within WSxM software.[38]
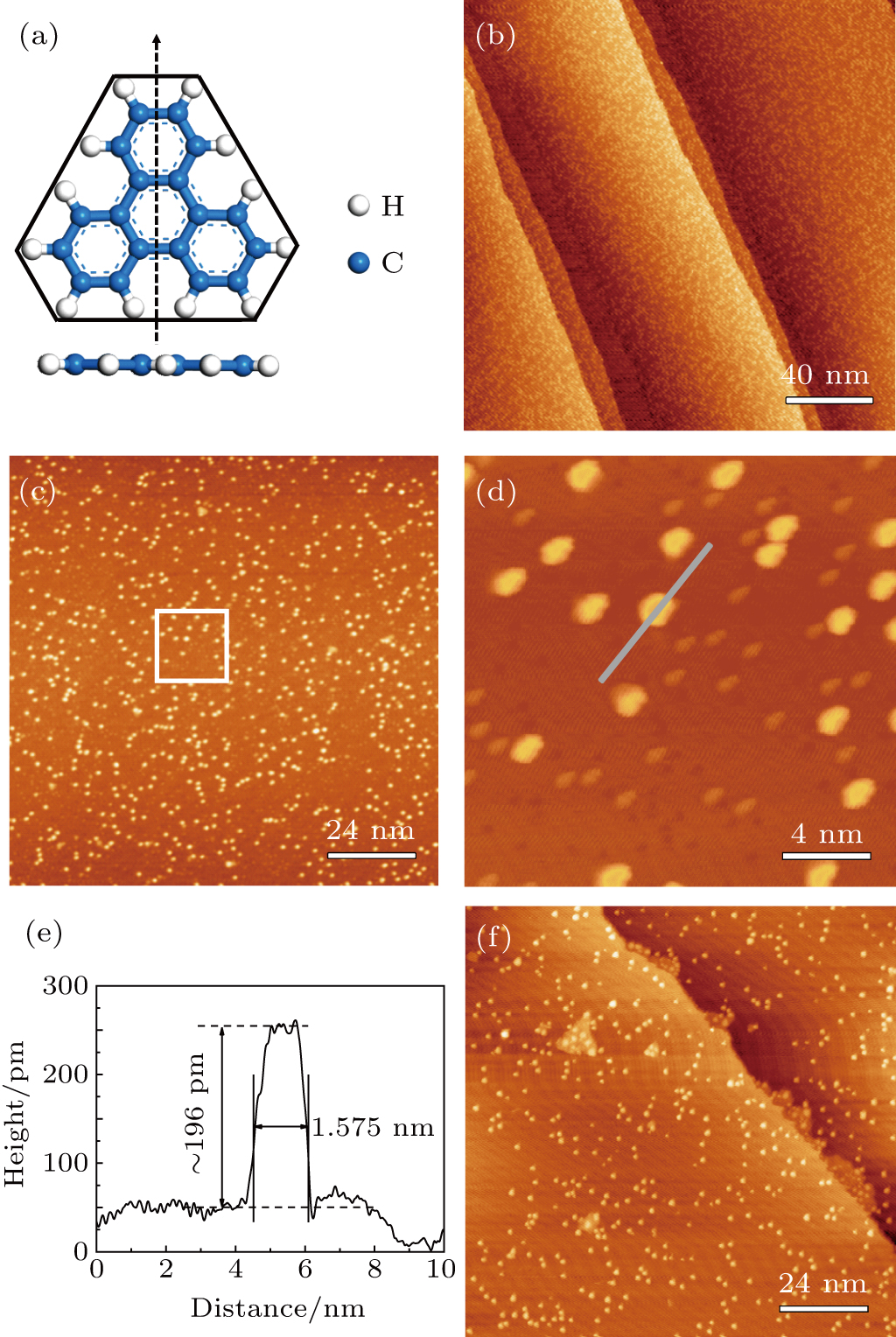
Figure 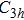
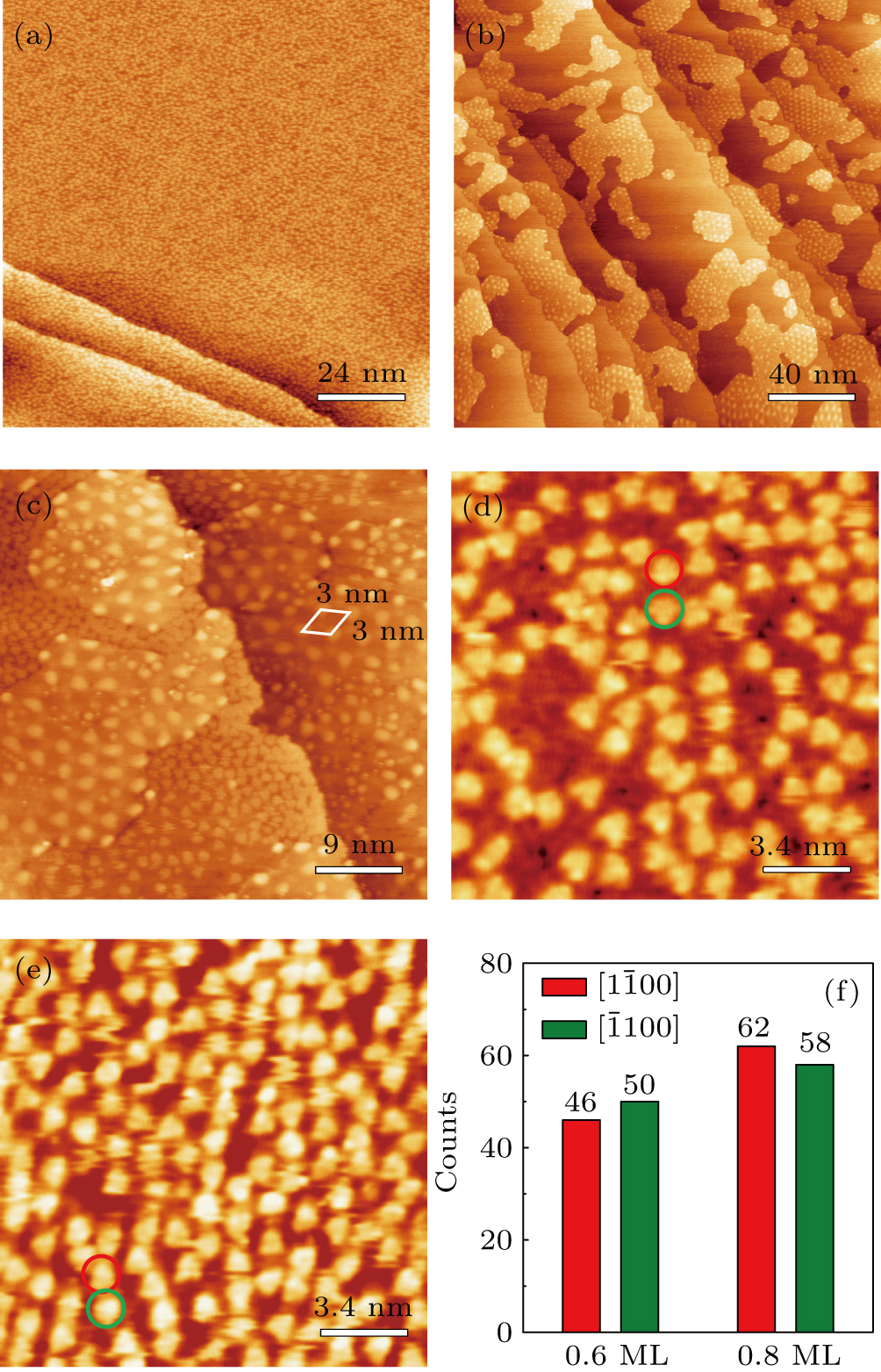
Figure 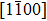
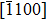
The observed two molecular axial orientations are mirror-symmetric to each other, which corresponds to a 60° rotation from each other. The array orientation of TP is largely dominated by the Ru(0001) surface. DFT simulation suggested TP on the Pt(111) surface has a preference for a hollow site.[46] Experimental results also showed that TP dominantly takes up the face-centered cubic (fcc) site and then occupies the hexagonal close-packed (hcp) site on the reconstructed Au(111) substrate.[47] It is proposed that the mutual coupling of the 3-fold symmetries of TP molecule and the substrate jointly requires TP occupying a 3-fold site of Ru(0001). Thus, the smallest energy-equivalent rotation is by 60°, equaling a reflection of TP.
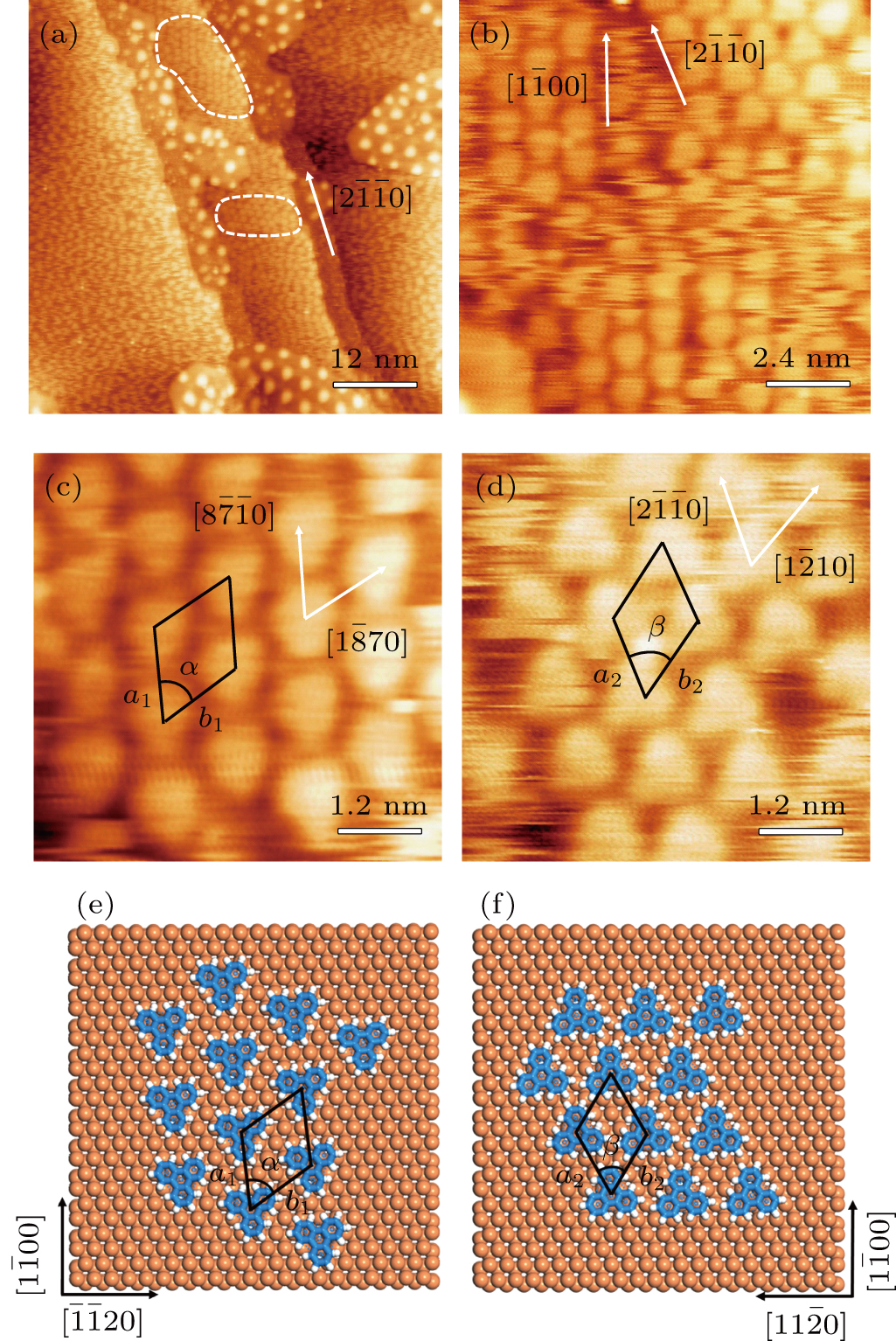
Figure
The histogram in Fig.
Upon formation of graphene islands on Ru(0001), TP molecules were further deposited onto the substrate. Figure 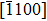
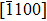
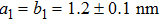
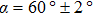
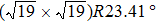
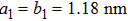
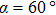
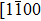
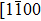
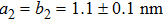
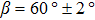
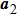

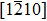
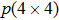
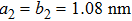
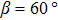
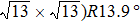
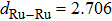
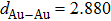
The molecule–substrate and molecule–molecule interactions both play an essential role in determining the molecular growth behavior in the monolayer regime. Stronger molecule–substrate interaction will facilitate amorphous growth. For relatively weaker molecule–substrate interaction, the laterally attractive molecule–molecule interaction will favor island growth at submonolayer stage if diffusion of molecules is not constrained. While the laterally repulsive (weak) intermolecular interaction will usually give rise to 2D liquid-like growth at submonolayer and form ordered structures at monolayer. For example, PTCDA and DM-PBDCI on noble metal surfaces exhibit island growth due to the attractive intermolecular interaction.[51,52] For 1,3,5-triphenylbenzene on Ru(0001), the adsorption energy of the bimolecular system is larger than that of the monomolecular system with DFT calculations, leading to molecular close-packed behavior.[45] However, in the present case, TP molecules on Ru(0001) at low coverage are hardly subject to the ordered structure even if being annealed to higher temperature and only at monolayer can ordered structures occur. Besides, the close-packed domains are more stable than the loosely packed disordering during the scanning (see Fig.
TP molecules lie flat on the Ru(0001) surface and have a predilection for 2D liquid-like growth mode. They adopt two adsorption configurations with one-to-one ratio of orientational distribution at submonolayer, which are energetically equivalent due to the three-fold symmetries of TP and the substrate. The TP overlayer on Ru(0001) can be converted into graphene nanostructures at 700 K and the grooves formed by graphene islands have confinement effect on the lateral movement of TP molecules due to weak interaction between TP and graphene islands supported by Ru(0001). At TP coverage close to 1.0 ML, two small close-packed domains with a 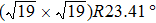
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 |