† Corresponding author. E-mail:
It is widely accepted that helium (He) bubbles can prevent dislocations from moving and causing hardening and embrittlement of the material. However, He can affect the mechanical properties of materials in various ways. In this work, ultrafine nanocrystal high entropy oxide (HEO) films with He implantation are prepared by using a radio frequency (RF) reactive magnetron sputtering system to investigate the effects of He bubbles located at grain boundary on the mechanical properties of the films. The mechanical properties of the HEO films are investigated systematically via nanoindentation measurements. The results indicate that the grain boundary cavities induced by He implantation can degrade the hardness, the elastic modulus, and the creep resistance of the HEO films. The mechanical properties of the HEO films are sensitive to the interaction between the He bubbles and the dominating defects.
High entropy alloys (HEAs) have been rapidly developed as a promising functional and structural material due to their outstanding properties, such as high mechanical properties and excellent corrosion resistance.[1–3] In recent years, the concept of entropy stabilization for HEAs materials has also been extended to the composite oxides,[4] termed high entropy oxides (HEOs), which also exhibit remarkable performance including colossal dielectric constants,[5] excellent ionic conductivity,[6,7] and high temperature stability.[8,9] HEOs are even expected to be used as radiation tolerance materials or tritium permeation barriers in nuclear reactors, considering the outstanding radiation tolerance of high-entropy materials and the huge permeation reduction factor (PRF) of ceramic materials.[10–12]
In nuclear reactors, neutron bombardment can generate considerable concentration of He in the materials via nuclear reactions.[13] He atoms are insoluble in materials and thus segregate easily into He bubbles.[13–16] Many previous reports have shown that the He bubbles can suppress the dislocation gliding by pinning the dislocations to cause material to harden even at low concentration.[17,18] However, it should be noted that He bubbles can cause material to soften by increasing the free volume to facilitate the formation of the shear bands when they segregate into glassy phase.[19,20] He bubbles can also enhance the interface sliding by reducing the interfacial cohesive energy when they segregate along the interface between the multilayer films.[21] This indicates that He bubbles can have various effects on the mechanical properties of materials. So far, the mechanism of the He effects on the mechanical properties of the materials has not been fully elucidated by conventional dislocation pinning theory, which is mostly due to the complicated interaction between the He bubbles and the material defects, and, more importantly, the behavior of the material deformation strongly depends on the material defect type. For example, the grain boundary, rather than the dislocations, plays a decisive role in the plastic deformation of the ultrafine nanocrystal material whose grain diameter is less than 10 nm,[22] while the dislocation motion dominates the plastic deformation of the coarse crystal material because a lower energy is required by the grain boundary sliding and the grain boundary atom diffusion than by the dislocation proliferation.[23,24] Therefore, He bubbles should have deep effects on the mechanical properties of the ultrafine nanocrystal materials, which have been explored as alternative materials for nuclear reactors,[25] because He bubbles are favorable for being segregated at the grain boundaries when they are introduced.[26] However, we are still unable to accurately describe the effects of He bubbles at the grain boundary on the mechanical properties of the ultrafine nanocrystal materials, even though some theoretical and experimental efforts were devoted to understanding the effects of He on the mechanical properties of these materials.[27,28]
In this work, the (Al–Cr–Fe–Ni)O ultrafine nanocrystal HEO films with different He concentrations are prepared by a radio frequency (RF) reactive magnetron sputtering system under different He partial pressures. The ultrafine nanocrystals are easily formed in the HEO materials due to the sluggish diffusion effect of the elements in high entropy materials. The mechanical properties of the HEO films are systemically investigated by a nanoindentation test. Changes of the hardness, reduced elastic modulus and creep resistance of the ultrafine nanocrystal HEO films have been analyzed.
(Al–Cr–Fe–Ni)O high-entropy oxide (HEO) films with He implantation were deposited on single crystal Si (100) substrates by an RF reactive magnetron sputtering system. Several circular aluminum, chromium, iron and nickel sectors (99.995% in purity and 1 mm in thickness) were combined into a sputtering target. The distance between the target and the substrate was maintained at 50 mm. The silicon substrates were cleaned ultrasonically with alcohol prior to being placed on the substrate holder. HEO films with He implantation were deposited in a stainless-steel vacuum chamber, and the basic pressure was 8.0×10−4 Pa prior to deposition. The target area was pre-sputtered for 20 min to remove impurities from the surface. A pure titanium (Ti) interlayer with a thickness of about 100 nm was deposited on the Si substrates prior to the deposition of HEO films. During the deposition of the HEO films with He implantation, pure Ar, O2, and He were introduced into the chamber. The concentration of He introduced into the films was controlled by changing the partial pressure of He in a range of 0.1 Pa–0.3 Pa during deposition. The RF power was maintained at 100 W. With a similar procedure, the (Al–Cr–Fe–Ni)O HEO film was also deposited on the single crystal Si (100), which serves as a control sample. During the deposition of the (Al–Cr–Fe–Ni)O HEO film, pure Ar and O2 were induced into the chamber. The rotation rate of the substrate holder was set to be 10 r/min to ensure elements uniformly distributed in the films, and the substrate was neither heated nor cooled intentionally during the deposition of the films. The thickness values of all of the films were kept at approximately 800 nm.
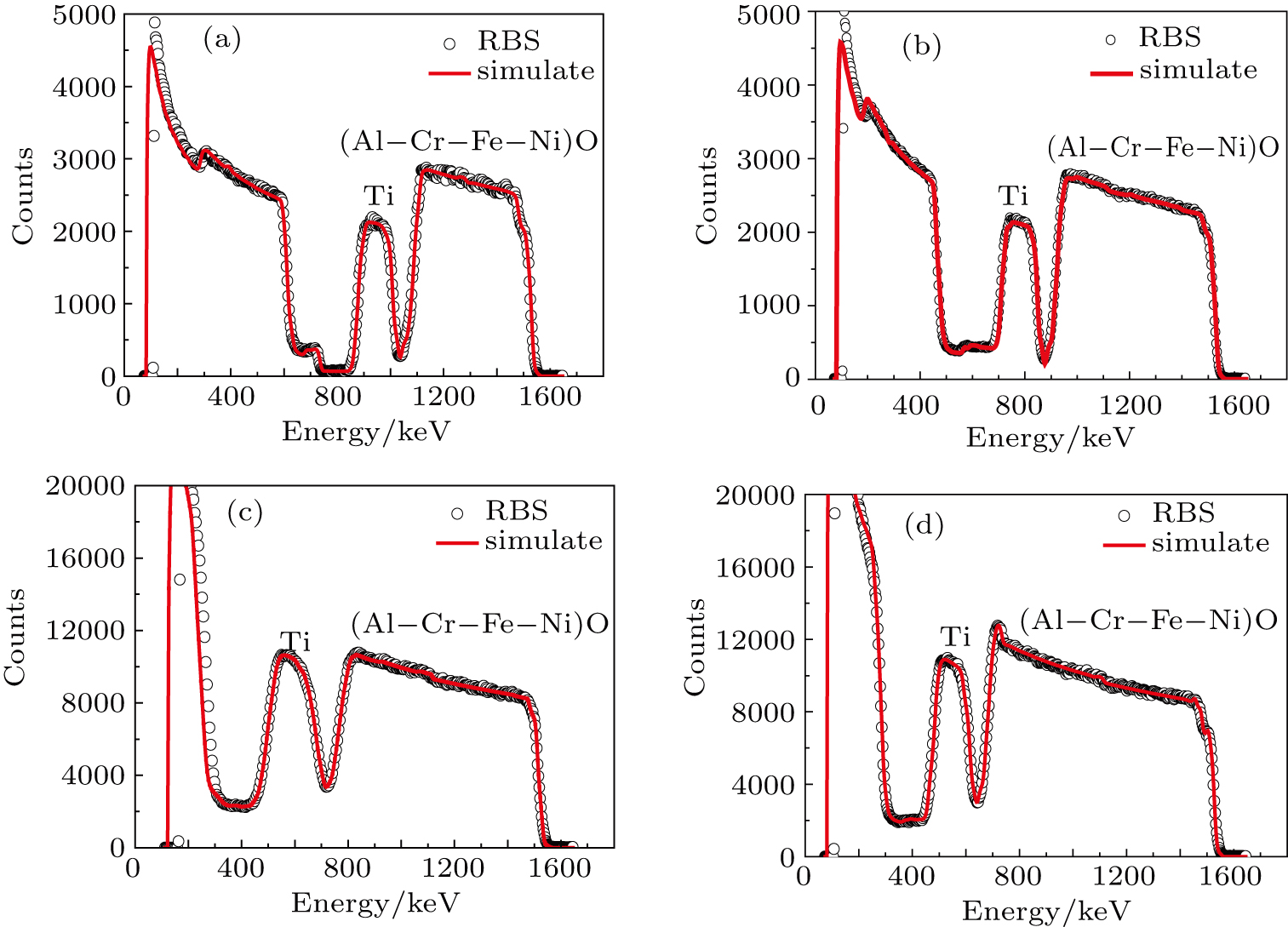
Rutherford backscattering spectrometry (RBS) was used to investigate the elemental compositions of the films with 2-MeV 
A nanoindentation instrument (NanoTest Vantage) equipped with a Berkovich indenter was employed in ambient conditions to evaluate the mechanical properties of these films. The samples were cut into square slices each with a side length of 5 mm and stuck on the sample stand with cyanoacrylate adhesive prior to nanoindention tests. The adopted maximum load and the load rate were 6 mN and 0.5 mN/s during indenting, respectively. Peak loads were chosen such that the percentage of the penetration depths to the film thickness varied from 13%–15%, a range in which the substrate effect can be neglected.[29] Once the maximum load had been reached, the load was fixed and held for 50 s. In that process, the load-displacement and displacement-time curves were recorded simultaneously. The values of hardness and elastic module of the samples were derived from the load-displacement data according to the Oliver-Pharr method.[30] The displacement-dwell time curves can reflect the creep behavior of the films.[31] To avoid experimental error, each test was repeated 10 times.
The RBS spectra and the simulated results of the as-deposited and He-implanted films are shown in Fig.
The evolutions of the surface morphology of the HEO films are shown in Fig.
 | Fig. 2. Surface morphology of (a) the as-deposited HEO film, (b) HEO films with 0.1-Pa He, (c) 0.2-Pa He, and (d) 0.3-Pa He implantation. |
It is also noted that with the increase of the implanted He content, the number of blisters decreases while the size of the blisters increases significantly. For the film with 0.3-Pa He implantation, the diameter of blisters can reach ∼300 nm. This indicates that the implanted He can speed up small blisters coalescing and forming large blisters.
As described above, the surface morphology of the film is strongly related to the behaviors of the implanted He. Zheng et al.[32] pointed out that He can be introduced into the films by sputtering deposition and homogeneously. Cheng et al.[33] suggested that He bubbles can be formed in the Ti film once the atomic ratio of He/Ti exceeds 3.7 at.%. With the accumulation and growth of He bubbles at higher He concentration, surface blisters can be formed on the film surface.[34] In this work, the He concentration of the HEO films is greater than 9 at.%, which thus contributes to the formation of the He bubbles, and the change of the surface morphology with He implantation can be attributed to the aggregation and evolution of the He bubbles.
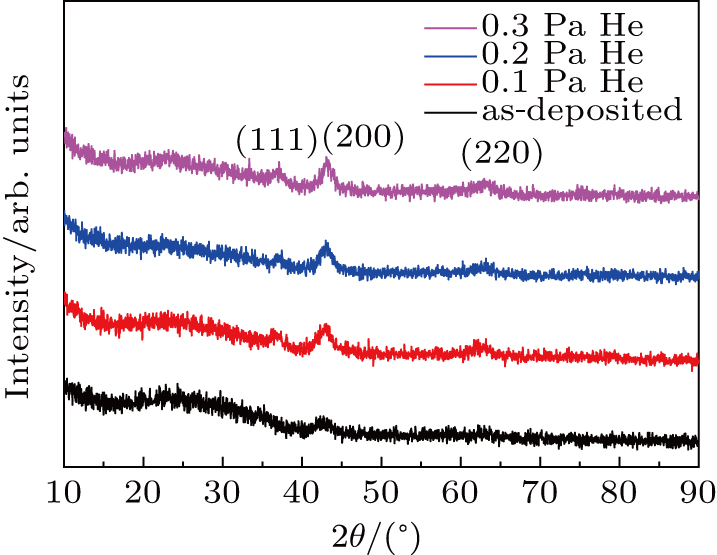
The GIXRD patterns of the HEO films are shown in Fig.
It is interesting to find that although the intensity of the (200) peak increases when He is introduced, two additional diffraction peaks of (111) and (220) located at 36.8° and 63.1° can also be found, respectively. These results show that the (200) preferential growth orientation of the HEO film is weakened when He is introduced. This can be attributed to the penning ionization which provides more ionized oxygen atoms to promote the formation of various textures when He atoms are introduced into the Ar–O2 mixed atmosphere.[35] It should be mentioned that the (200) diffraction peak of the He-implanted HEO film shifts slightly to larger angle than that of the as-deposited HEO film. It indicates that the interplanar spacing of the HEO films decreases because of the He implantation. Moreover, the average grain size of the HEO film obtained from the GIXRD pattern (Fig.
| Table 1.
Average grain sizes of the HEO films. . |
The force-displacement curves of the HEO films measured by nanoindentation system are shown in Fig.
The plots of average hardness (H) and reduced elastic modulus (Er) of the HEO films versus He partial pressure are shown in Fig.
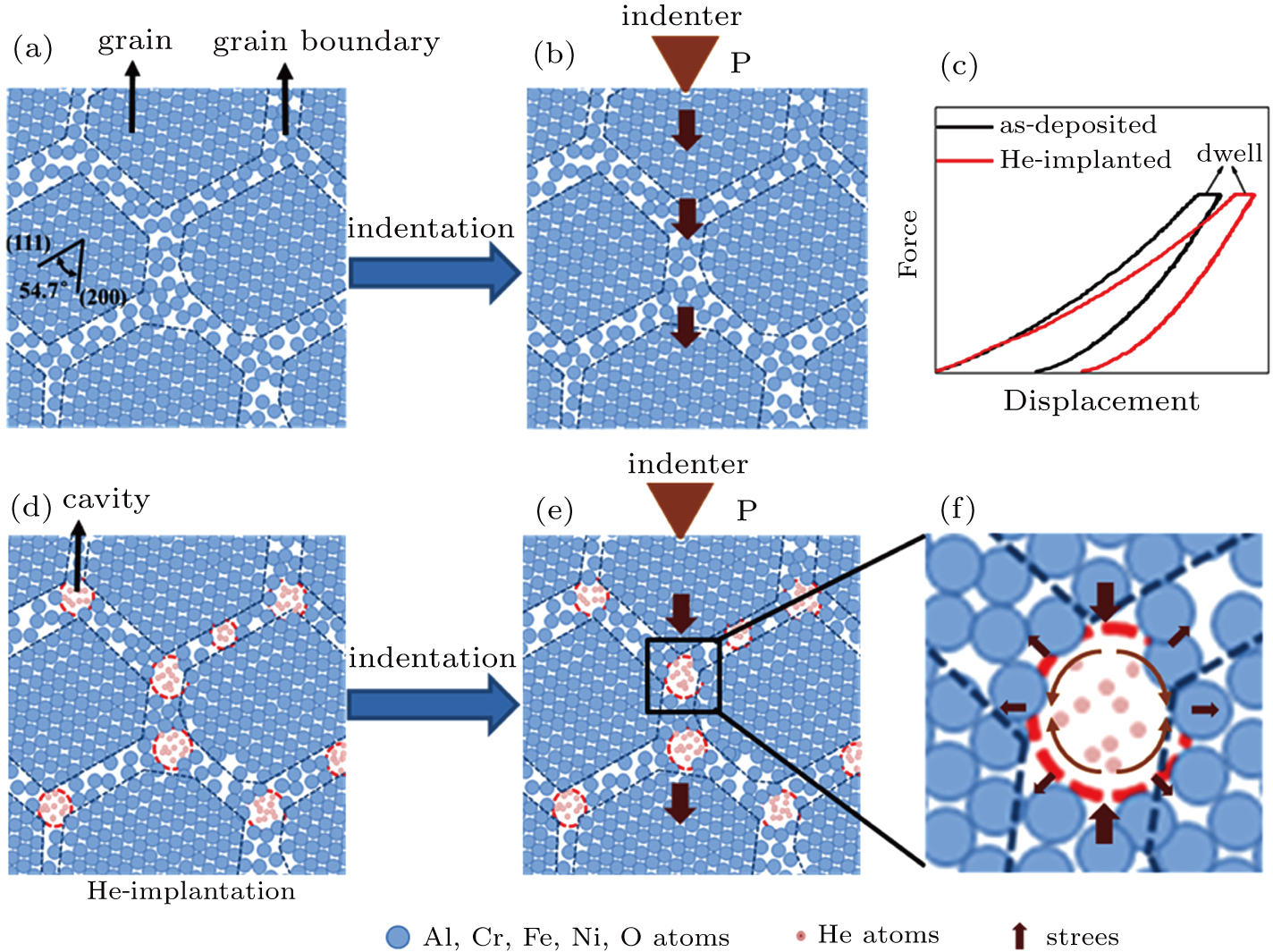
Suzudo et al.[27] suggested that He bubbles can result in the formation of grain boundary cavities. The higher He content in the HEO film can expedite the formation of the grain boundary cavities. As the grain boundary cavities can disrupt the chemical bonds to form low-cohesive energy grain boundaries and reduce the resistant volume to mechanical stresses, the H and Er continue to decrease with the as the size and density of the cavities increase.[14,36] The H and Er deterioration caused by the grain boundary cavities can be described as flows:[37,38]
Figure
 | Fig. 7. (a) Normalized displacement-dwell time curves, and (b) ln(stain rate)–ln(stress) curves of HEO films. |
The diffusion creep, which is achieved mainly by Coble creep and Nabarro–Herring creep, is favorable for nanocrystal materials due to the fine grain size.[40] Atoms or vacancies can diffuse through the grains in Nabarro–Herring creep, while atoms or vacancies diffuse along grain boundaries in Coble creep. Coble creep is more likely to occur than Nabarro–Herring creep when the operate temperature is less than half the melting point (0.5Tm) as the activation energy required for Coble creep is less than that for Nabarro–Herring creep.[40] Therefore, Coble creep is the dominant mechanism for the ultrafine nanocrystal HEO films during nanoindentation creep at ambient temperature.
According to the general Norton relationship, the creep strain rate (
The effects of the grain boundary cavities induced by He implantation on the creep resistance of the HEO film are depicted schematically in Fig.

In this study, the (Al0.31Cr0.20Fe0.14Ni0.35)O HEO films with various He content are synthesized by magnetron sputtering. The microstructure analysis indicate that the (200) preferred orientation of the HEO films becomes weak when He is introduced into the films due to the higher ionization rate of oxygen in the deposition. The hardness, reduced elastic modulus and creep behavior for each of the HEO films are investigated with a nanoindentation system. It is found that the grain boundary cavities induced by He implantation can have a significant influence on the mechanical properties of the HEO films. The mechanical properties of the HEO films with He implantation depend not only on the properties of the films themselves but also on the interaction between the He bubbles and the defects that dominate the plastic deformation of the material.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] |