Project supported by the National Key Basic Research Program of China (Grant No. 2014CB932400), the National Natural Science Foundation of China (Grant No. 51772167), the China Postdoctoral Science Foundation (Grant No. 2016M591169), and the Shenzhen Municipal Basic Research Project, China (Grant No. JCYJ20170412171311288).
Project supported by the National Key Basic Research Program of China (Grant No. 2014CB932400), the National Natural Science Foundation of China (Grant No. 51772167), the China Postdoctoral Science Foundation (Grant No. 2016M591169), and the Shenzhen Municipal Basic Research Project, China (Grant No. JCYJ20170412171311288).
† Corresponding author. E-mail:
Project supported by the National Key Basic Research Program of China (Grant No. 2014CB932400), the National Natural Science Foundation of China (Grant No. 51772167), the China Postdoctoral Science Foundation (Grant No. 2016M591169), and the Shenzhen Municipal Basic Research Project, China (Grant No. JCYJ20170412171311288).
Hybrid liquid/solid electrolytes (HLSEs) consisting of conventional organic liquid electrolyte (LE), polyacrylonitrile (PAN), and ceramic lithium ion conductor Li1.5Al0.5Ge1.5(PO4)3 (LAGP) are proposed and investigated. The HLSE has a high ionic conductivity of over 2.25 × 10−3 S/cm at 25 °C, and an extended electrochemical window of up to 4.8 V versus Li/Li+. The Li|HLSE|Li symmetric cells and Li|HLSE|LiFePO4 cells exhibit small interfacial area specific resistances (ASRs) comparable to that of LE while much smaller than that of ceramic LAGP electrolyte, and excellent performance at room temperature. Bis(trifluoromethane sulfonimide) salt in HLSE significantly affects the properties and electrochemical behaviors. Side reactions can be effectively suppressed by lowering the concentration of Li salt. It is a feasible strategy for pursuing the high energy density batteries with higher safety.
Lithium ion battery (LIB) has significantly revolutionized our world since its appearance.[1] Further advancement of LIB requires the electrode materials with high energy densities.[2] Lithium metal is an ideal anode choice because of its extraordinarily high theoretical specific capacity (3860 mAh/g), the lowest redox potential (−3.04 V), and the lightest weight (0.59 g/cm3). However, non-uniform deposition, large volume variation and dendrite formation lead to low Coulombic efficiency, shortened lifespan, and serious safety in organic liquid electrolyte when Li metal is used.[3,4] All solid-state batteries adopting solid-state electrolytes are considered to be a promising solution.[5–9] Nevertheless, poor solid-solid contact between electrolyte-electrode interfaces and particles in electrodes result in such large interfacial resistance that all solid-state batteries can hardly work.[10] Strain and stress produced during cycling further aggravate this dilemma.[11] Many efforts were devoted to improving the electrode-electrolyte interfacial contact. Modifying the surface of the ceramic electrolyte or electrode surface can effectively reduce the interfacial resistance but it only partially solves the problem, and is still far from practical application.[12–20] A hybrid electrolyte consisting of two or more kinds of electrolyte systems may possess the merits of each component and even novel properties bring new electrolyte options for advanced batteries. Maier et al. proposed a soggy sand electrolyte using liquid electrolyte and inert oxide particles (Al2O3, TiO2, SiO2, and so on), which can be adjusted from a liquid electrolyte to a gel-like soft electrolyte; the soggy sand electrolytes exhibited novel properties and displayed promising performances.[21–23] A hybrid of liquid electrolyte with polymer is the widely studied and used gel electrolyte.[24] A combination of ceramic and polymer is another type of hybrid electrolyte that nowadays many researchers are working on, yet it still does not satisfy practical applications.[20,25,26]
Recently, we proposed a new strategy of using a composite hybrid liquid/solid electrolyte (HLSE), which consists of a conventional organic liquid electrolyte (e.g., 1-M LiTFSI-EC/PC), widely adopted polymer (e.g., polyacrylonitrile, PAN), and a ceramic lithium ion conductor (e.g., Li1.5Al0.5Ge1.5(PO4)3, LAGP), prepared through a simple procedure. Liquid electrolyte (LE) in the electrolyte ensures high ionic conductivity and intimate contacts between electrolyte and electrodes. The polymer endows the electrolyte with a semi-solid state, extending the electrochemical window and flexibility. The ceramic lithium ion conductor in HLSE further extends the electrochemical window (up to about 4.8 V), which is suitable for most conventional cathodes including LiFePO4 and LiNixCoyMnzO2 (0 < x, y, z < 1, x + y + z = 1). The ceramic lithium ion conductor may provide extra ion transport pathways,[27] and reduce side reactions. Employing polymer and ceramic in the HLSE will reduce the LE content in the electrolyte/batteries, which will enhance the safety of batteries (see Fig.
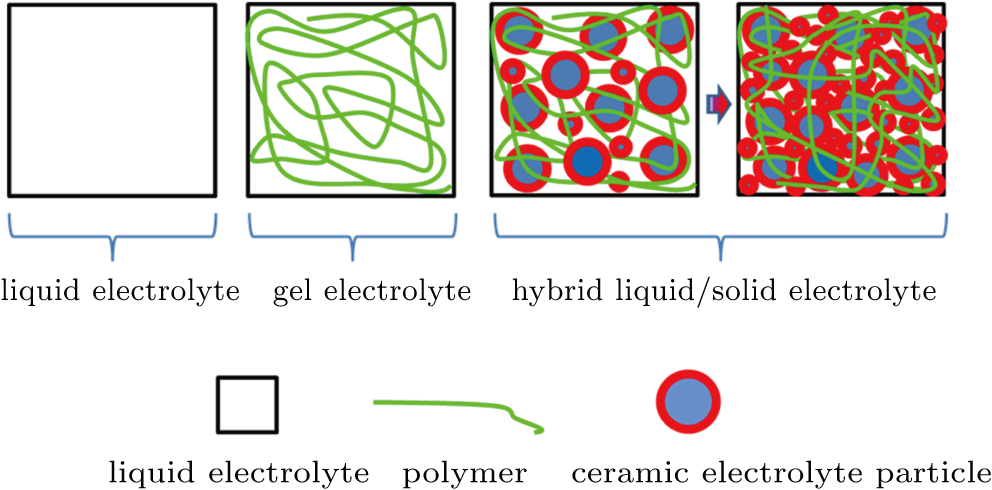 | Fig. 1. (color online) Schematics of electrolyte evolution from liquid toward hybrid liquid/solid state. |
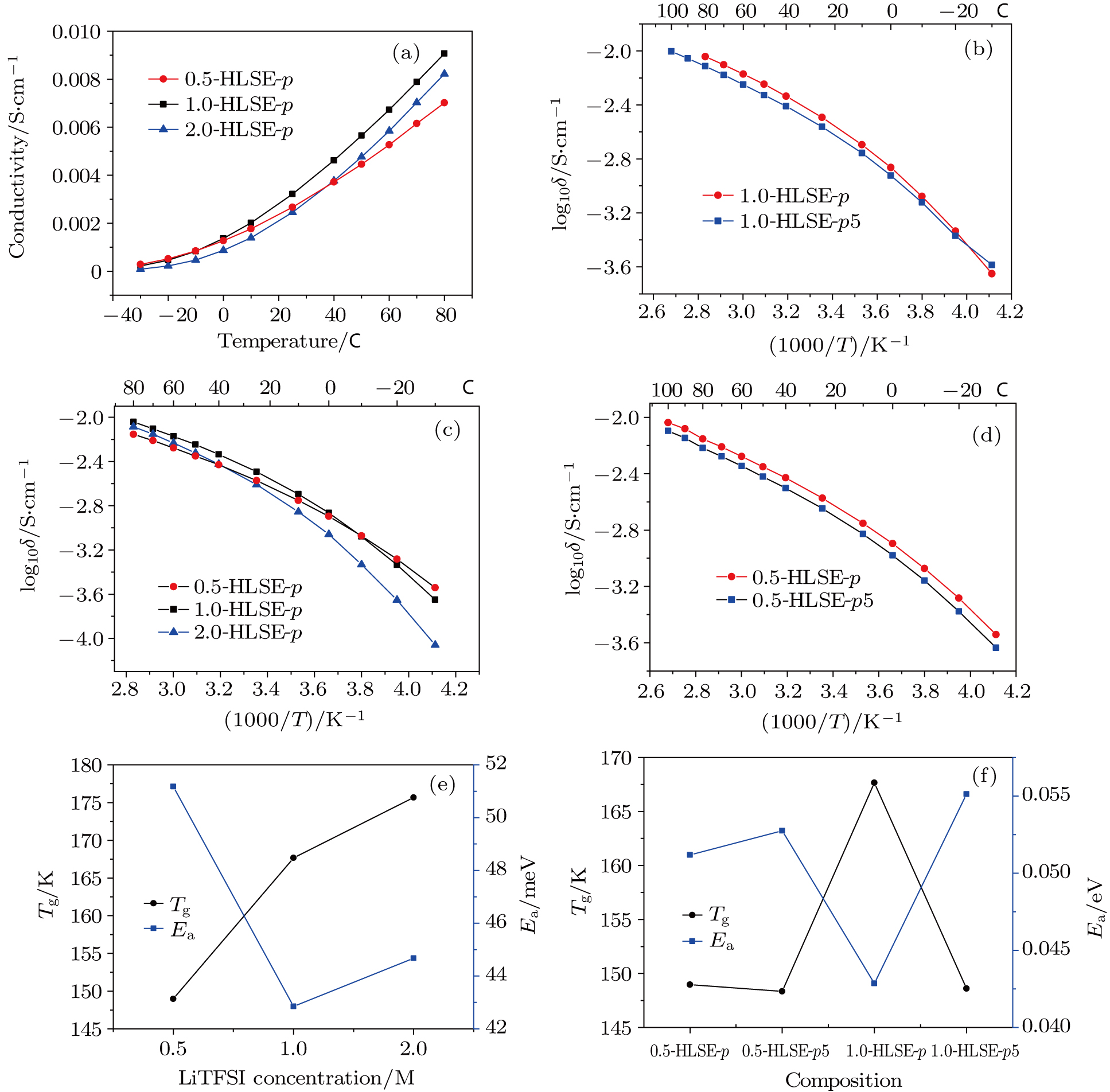
Here, we report the novel properties of the HLSEs with excellent electrochemical performance by using a lower concentration of Li salt. The conductivity of the HLSE is over 2.25 × 10−3 S/cm at room temperature, comparable to that of a conventional liquid electrolyte. The HLSEs have extended electrochemical stable windows of up to 4.8 V versus Li/Li+. Li|HLSE|Li symmetric cells and Li|HLSE|LiFePO4 cells exhibit small interfacial area specific resistances (ASRs), which are comparable to that of LE, much smaller than that of ceramic LAGP electrolyte, and show very small polarization and stability at room temperature (RT).
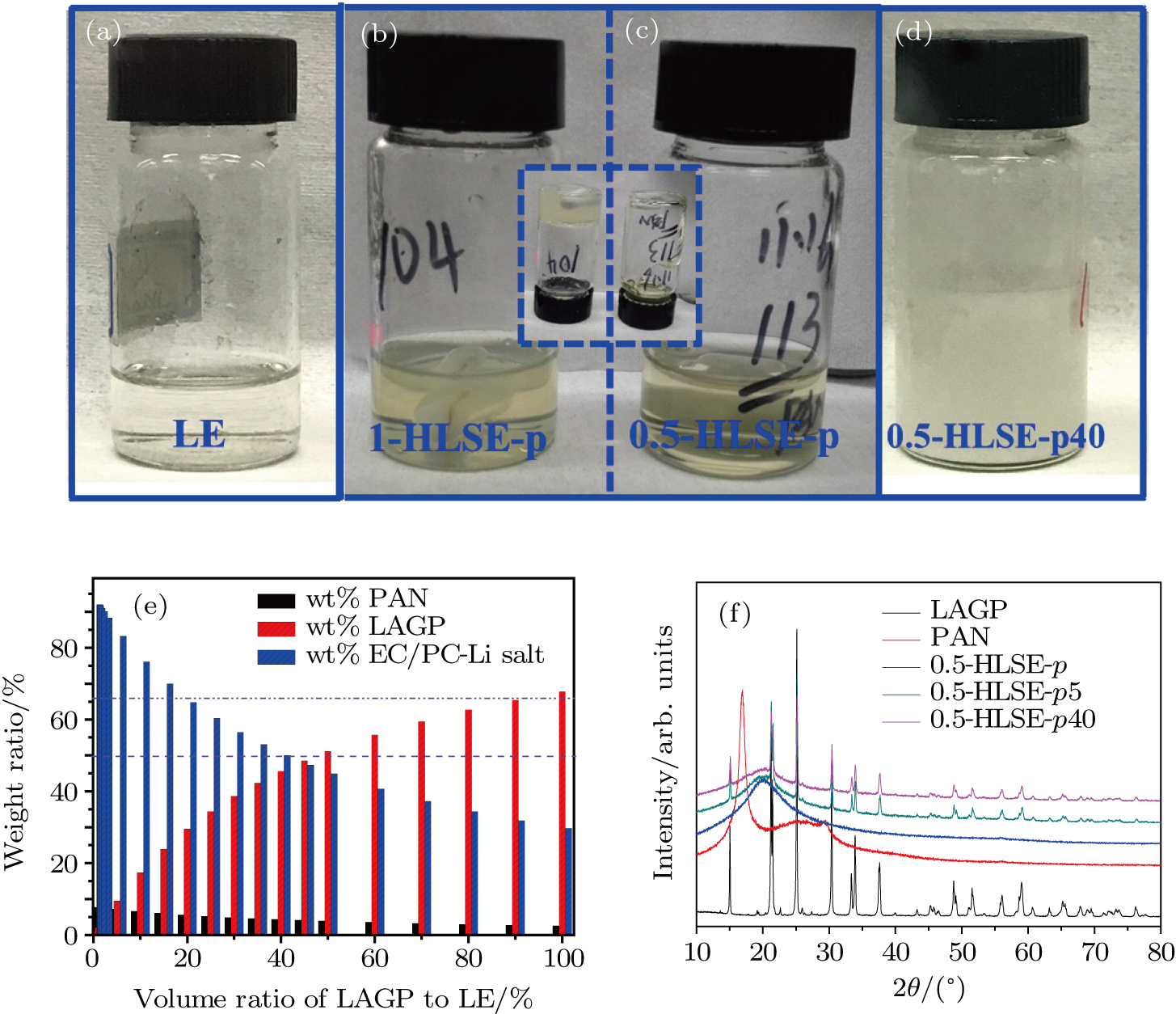
Ethylene carbonate (EC, anhydrous 99.0%, Aldrich) and propylene carbonate (PC, anhydrous 99.7%, Aldrich) were first mixed (1:1 vol.) to form the solvent. Then bis(trifluoromethane sulfonimide) (LiTFSI, dried at 80 °C under vacuum prior to use) was dissolved in the above solvent to obtain 0.5 M–2 M LiTFSI-EC/PC based liquid electrolyte (denoted as LE hereafter, water content < 20 ppm). Polyacrylonitrile (PAN, Mw ∼ 1.5 × 105 purchased from Macklin, dried at 80 °C for 24 h under vacuum before use) and Li1.5Al0.5Ge1.5(PO4)3 (LAGP, purchased from Shenzhen MTI Corp.) were added to the base liquid electrolyte according to the required amount and vigorously stirred at 110 °C to form a homogeneous composite electrolyte. The obtained electrolytes were then coated onto the air laid paper consisting of 45% polyester and 55% cellulose fibers by simply immersing the air laid paper into the electrolyte, forming the composite separator after cooling down. All these processes were carried out in an argon filled glove box with H2O < 0.1 ppm and O2 < 0.5 ppm. There were two kinds of HLSE electrolytes. The first kind was prepared with only 8-wt% PAN addition to the base liquid electrolyte (LE), and the second one contained x-vol% LAGP (0 < x < 70) addition besides the 8-wt% PAN. They are abbreviated as y-HLSE-p and y-HLSE-px respectively for simplicity unless otherwise specified hereafter, where y indicates the concentration of LiTFSI, p indicates PAN and x denotes the volume ratio of LAGP to base LE, respectively.
The ionic conductivity of electrolytes was examined using a custom-made toolkit calibrated using standard potassium chloride (KCl) aqueous solution. The electrochemical window (Ewind) of the electrolyte was examined using the Li|Electrolyte|SS (stainless steel) configuration from 2.5 V to 5 V with a scan rate of 1 mV/s.
To prepare the LiFePO4 cathode, LiFePO4, carbon black and PVDF (80:10:10 by weight) were first mixed thoroughly using N-methyl pyrrolidone (NMP) solvent. Then the obtained slurry was spread evenly on aluminum foil to produce the green cathode film with active material loading of ∼ 5 mg/cm−2, the cathode films were then dried at 110 °C for 12 h under a vacuum prior to use.
The CR2032 coin cells were assembled in an argon filled glove box with lithium metal anode, LiFePO4 cathode, and the HLSE electrolyte. The charge/discharge cycling was performed on a LAND BT2000 battery tester.
Figure
Figure
Figure
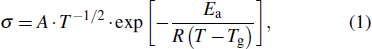 |
The ionic conductivity further drops when ceramic lithium ion conductor LAGP was introduced into 1.0-HLSE-p5 (Fig.
| Table 1.
Ionic conductivities of HLSEs (in units mS/cm). . |
We further investigate their electrochemical behaviors as the 0.5-M system electrolyte shows different behavior from the others. The electrochemical stable window of the hybrid liquid/solid electrolyte extends to a higher potential with the addition of LAGP as shown in Fig.
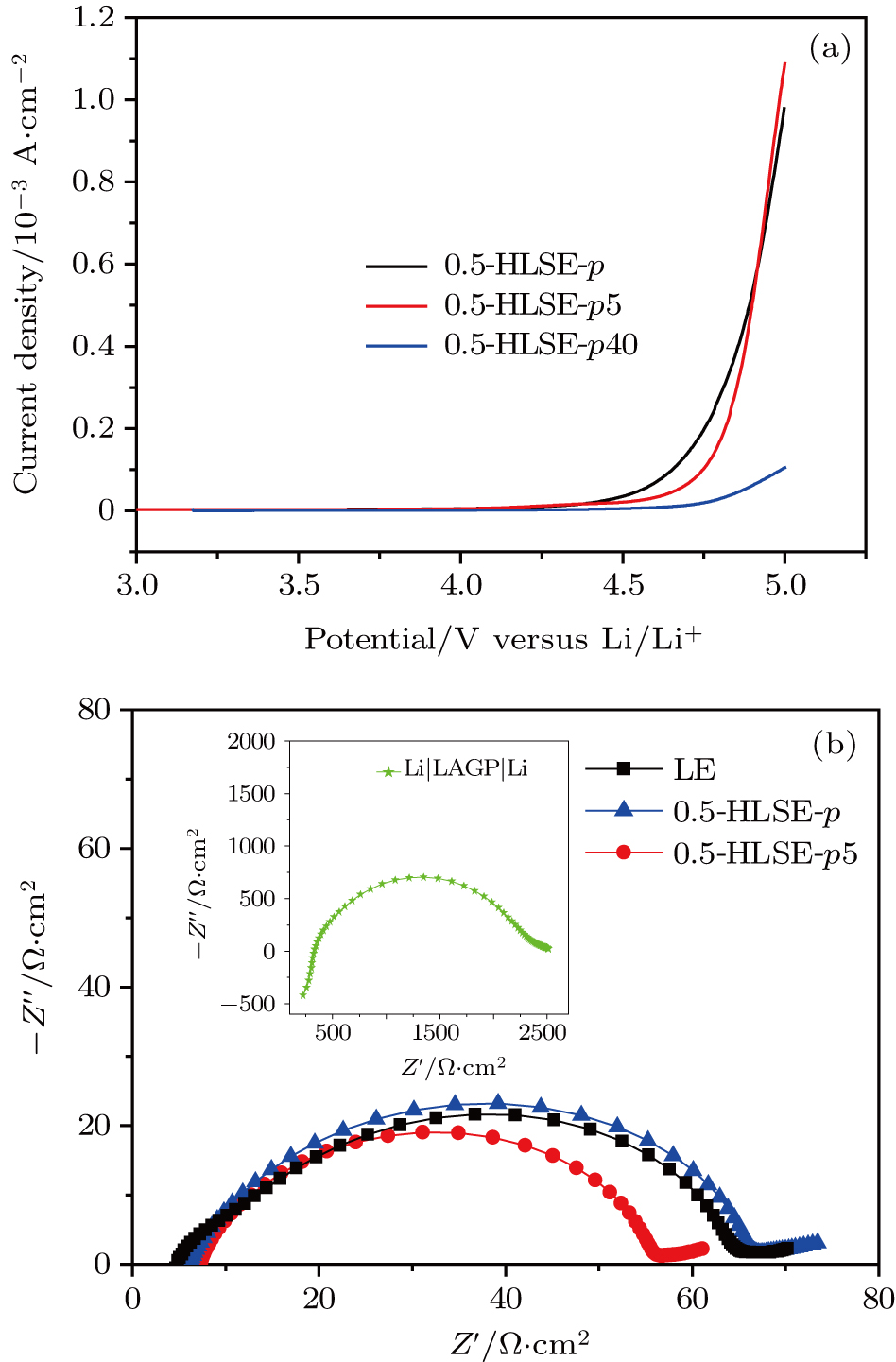 | Fig. 4. (color online) (a) Plots of current density versus potential for the cases of 0.5-HLSE-p, -p5, and –p10, and (b) Nyquist plots of interfacial ASR between Li metal and electrolytes. |
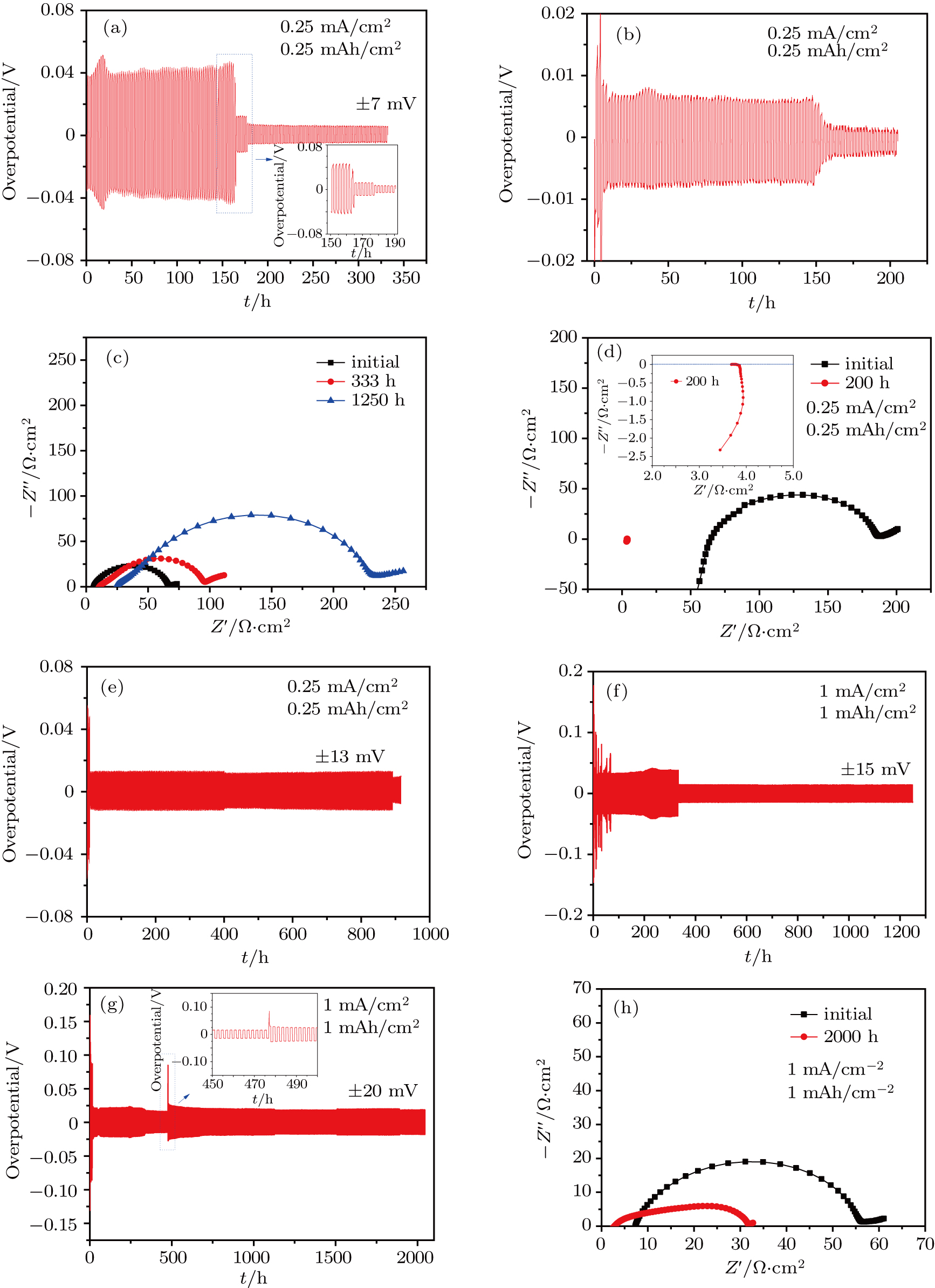
For the present reported solid-state batteries, the extremely large interfacial resistance limits their practical applications.[10] We evaluate the interfacial resistances between the metal Li anodes and the HLSEs using the symmetric cell. The ASRs are very close to that between the Li anodes and the liquid electrolyte as observed Fig.
We also investigate the electrochemical behavior of the HLSE (0.5-HLSE-p5) using the Li symmetric cell with an electrolyte sandwiched between two metallic lithium. As shown in Fig.
Figure
 | Fig. 6. SEM images of surface Li anode of (a) Li|0.5-HLSE-p|Li after 1250 h, and (b) Li|0.5-HLSE-p5| after 2000-h cycling at 1 mA/cm2 with a capacity of 1 mAh/cm2 in each half cycle. |
When adopted in the Li–LiFePO4 cell, the HLSEs exhibit unexpectedly small impedance compared with that in the 1-M LiTFSI EC/PC liquid electrolyte as shown in Fig.
It is worthwhile pointing out that for 1.0-HLSEs, robust SEI can be formed rapidly and effectively suppress side reactions only when a much greater amount of LAGP (40 vol%) is introduced (which will soon be published in our next work), which again verifies the importance of lithium salt concentration in HLSEs.
In this work, we report novel hybrid liquid/solid electrolytes (HLSEs) with excellent electrochemical performance by simply using a lower lithium salt concentration. The HLSEs show conductivities of over 2.25 × 10−3 S/cm at RT, comparable to that of a conventional liquid electrolyte, and extend electrochemical stable windows of up to 4.8 V versus Li/Li+. The lower LiTFSI concentration favors the suppression of the side reaction in HLSE. The Li|HLSE|Li symmetric cells and Li|HLSE|LiFePO4 cells exhibit small interfacial ASRs comparable to that of the liquid electrolyte while much smaller than that of the ceramic LAGP electrolyte, thus showing very small polarization, excellent rate and cyclic performance at room temperature.
The molecular mechanism of how the lithium salt affects the properties of the HLSEs needs further investigation. We can also change the content/composition of polymer and ceramic electrolyte to further reduce the LE content in the system, thus improving safety, which is useful in practical applications.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] |