† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Zhejiang Province, China (Grant Nos. LY16F040003 and LY16A040007) and the National Natural Science Foundation of China (Grant Nos. 51401069 and 11574067).
Atomic-layer MoS2 ultrathin films are synthesized using a hot filament chemical vapor deposition method. A combination of atomic force microscopy (AFM), x-ray diffraction (XRD), high-resolution transition electron microscopy (HRTEM), photoluminescence (PL), and x-ray photoelectron spectroscopy (XPS) characterization methods is applied to investigate the crystal structures, valence states, and compositions of the ultrathin film areas. The nucleation particles show irregular morphology, while for a larger size somewhere, the films are granular and the grains have a triangle shape. The films grow in a preferred orientation (002). The HRTEM images present the graphene-like structure of stacked layers with low density of stacking fault, and the interlayer distance of plane is measured to be about 0.63 nm. It shows a clear quasi-honeycomb-like structure and 6-fold coordination symmetry. Room-temperature PL spectra for the atomic layer MoS2 under the condition of right and left circular light show that for both cases, the A1 and B1 direct excitonic transitions can be observed. In the meantime, valley polarization resolved PL spectra are obtained. XPS measurements provide high-purity samples aside from some contaminations from the air, and confirm the presence of pure MoS2. The stoichiometric mole ratio of S/Mo is about 2.0–2.1, suggesting that sulfur is abundant rather than deficient in the atomic layer MoS2 under our experimental conditions.
A two-dimensional (2D) transition metal dichalcogenide (TMD) MX2, with M being a transition metal Mo or W, and X being a chalcogen, say S or Se, is attracting increasing interest for its great potential applications in the fields of nanoelectronics, optoelectronics, lithium batteries, hydrogen storage, and catalysis based on their novel nanoelectronic and optoelectronic properties.[1–3] In particular, 2D atomic MS2 ultrathin films were reported to have direct bandgaps of 1–2 eV and indirect-to-direct bandgap transition with reducing number of layers, leading to exotic electronic and optical properties.[4] Recently, it was proposed as a suitable material for valleytronics. Excitons and charged excitons (trions) can be created in TMD monolayers by optical excitation, as the use of circular polarized light was shown to produce long-lived valley polarization.[4–6] The strong emission from the direct gap structure of monolayer MoS2 also promises the applications in optoelectronics.[1]
TMD MoS2 materials have been known to be in the form of nested fullerene-like structure, consisting of a single layer of molybdenum atoms sandwiched between two layers of sulfur atoms in a trigonal prismatic structure.[7] Substantial efforts have been made to prepare layered MoS2 (extended to WS2),[8,9] such as scotch tape based micromechanical exfoliation, liquid exfoliation, physical vapor deposition, hydrothermal synthesis, thermolysis of single precursor containing Mo and S, chemical vapor deposition using MoCl2 or MoO3 and S as sources, laser-based thinning, plasma thinning and grapheme-assisted growth, where chemical vapor deposition (CVD) method is regarded as the most practical method of synthesizing large-area MoS2 nanosheets.[1] Large-area MoS2 films were synthesized on SiO2/Si substrates with CVD, using the sulfurization of MoO3 in the sulfur vapor assisted by the pretreatment for the substrates in a nitrogen environment.[1] In Ref. [10], a process for synthesizing the WS2 nanosheets through the sulfurization of an atomic layer deposition (ALD) WO3 film was described, which has systematic layer controllability and wafer-level uniformity. The limitations of previously reported methods were also mentioned in Ref. [10], such as difficulties in systematically controlling the thickness. With the help of the ability to control the thickness of the films more directly, highly homogenous TMD (MoS2) films were deposited on a large area by sulfurizing the pre-deposited thin Mo films.[11] Two steps after the thin film of Mo deposited were needed.[11] To our knowledge, it is still a challenge to synthesize the MoS2 ultrathin films in atomic layer with controllable thickness by using fewer processing steps.
Inspired by the reported successful research works, we adopt a simple hot filament chemical vapor deposition method by only one step to synthesize the MoS2 ultrathin films. The scale of millimeter square atomic-layer MoS2 films have been obtained, which has been confirmed by a thickness mapping measurement in our previous report.[12] In this paper, further investigations on the crystal structures, valence states, and compositions of the materials localized at an atomic layer area are carried out in order to confirm the production of the atomic layer MoS2. Regarding the future studies on the atomic layer MoS2, it is very important to investigate the preparation method in detail, because there may be something different in structure or composition among such thin layers obtained with different preparation methods.
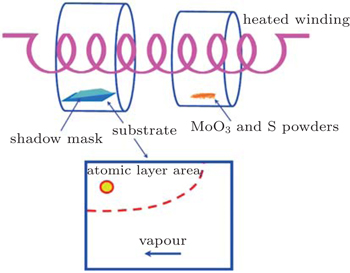
MoO3 and S powders mixed in a specific ratio were used as the reactants, in which MoS2 atomic layer can grow directly on substrates in one step by a hot filament chemical vapor deposition method. The reaction was done in a 30-mm-diameter horizontal quartz tube in vacuum. Specifically, a mixture of molybdenum trioxide (AR, 99.95%, Aladdin) and sulfur sublimed (Yongda) with a specific mole ratio of 1:49 (normally 0.003 g) was placed in the center of the tube as source. SiO2/Si(001) substrates were placed in another quartz tube near the heated winding downstream 2 cm away from the source material as the deposition acceptors. Before reaction began, the Si (001) substrates had been cleaned by the aqueous method and dried at 50 °C for half an hour. While deposition, the tube chamber was pumped into vacuum with a pressure lower than 0.1 kPa and flushed by Ar gas three times to remove the oxygen residue. Then, the winding was heated for deposition under 7 V bias condition for 90 s for growth. Finally, the tube was cooled down to room temperature for 30 min in vacuum. Figure
Microscopic and spectroscopic measurements aim to discover the structures, valence states, and concentration of the atomic layers MoS2. Tapping mode atomic force microscopy (AFM) experiments were performed in a multimode AFM microscope coupled to a micronano ChinaSPM controller (digital instruments). Commercially etched silicon tips with a typical resonance frequency of about 16 kHz were used as AFM probes. The crystal structures of the specimen were identified by x-ray diffraction (XRD) (DX-2500/2600) using CuKα radiation (λ = 0.1541 nm). Detailed lattice structures were obtained by a high-resolution transition electron microscopy (HRTEM) (Tecnai G2 F30) with an energy dispersive x-ray (EDAX) analyzer (DPP-II). In order to prepare the HRTEM specimen, the surface of ultrathin film was scratched by the TEM grids directly. In addition, the samples were characterized by transmission electron microscopy energy dispersive x-ray spectroscopy (TEM-EDX) to confirm whether the real stoichiometry meets the expectation. XPS (ESCALAB 250) was used to assess the chemical states of the thin films. All XPS spectra were corrected using the C 1s line at 284.6 eV. Photoluminescence (PL) spectra were measured with laser, which was operated at 405 nm wavelength, 5 mW power, and a large laser spot. We performed experimentally circularly polarized photoluminescence measurements on the atomic layer MoS2 and acquired circularly polarized PL spectra under the condition of right and left circularly polarized lights at room temperature in the case of off-resonance excitation state.
In order to show the specific features of the atomic layer MoS2 at the nucleation stage, the image of nucleation particles and the corresponding height profiles along a marked direction are shown in Fig.
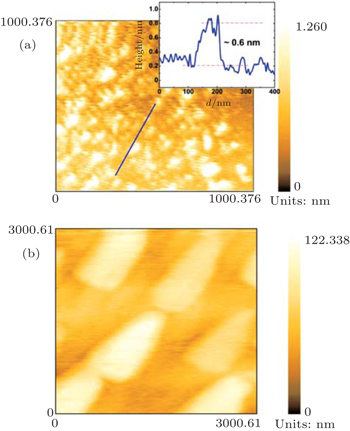 | Fig. 2. AFM images and the corresponding height profiles (insets) along the marked directions of the ultrathin films, in the nucleation area (a) and another area (b), respectively. |
Figure
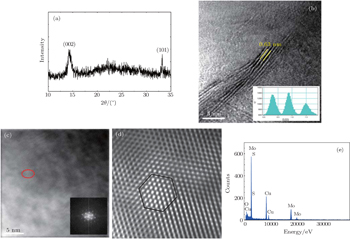
In order to investigate the crystal structures in detail, the HRTEM measurement is performed. We prepare the TEM specimen through scratching the surface of the selected area of the film using the TEM grids directly. Sometimes, a wedge-shaped HRTEM sample can be obtained, leading to the “quasi cross-section” HRTEM images. As shown in Fig.
Figure
TEM–EDX pattern of the region enclosed by the circle is shown in Fig.
PL spectrum studies offer a route to probe the thickness for an atomic layer of MoS2.[11] It has been reported that PL spectra show strong emission at the A1 and B1 direct excitonic transitions in monolayer crystal of MoS2, which are different from those of bulk MoS2 with the indirect band gap.[11] Under our experimental conditions, it is hard to focus exactly on the zone of the monolayer due to the limit of the equipment, because no micro-PL can be performed. Hence, a larger laser spot is irradiated to cover the atomic layer zone selected. Figure
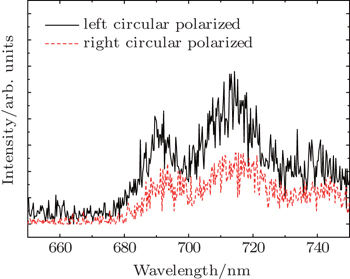 | Fig. 4. Room-temperature circularly polarized components of luminescence spectrum for the MoS2 atomic layers. |
In physical concerns, for a monolayer of MoS2, in the Brillouin zone, there exists the direct bandgap between the minimum of the conduction band and the maximum of the valence band at the k and k′ points (two generate valleys). A transition appears from direct bandgap of monolayer MoS2 to indirect bandgap with increasing layer number. The competition between direct and indirect electronic transitions dramatically reduces the PL quantum efficiency.[16] Thus, experimentally the PL spectrum intensity decreases with increasing layer number, until no emission is observed.[11,17] Under our experimental conditions, relatively high intensities of the two peaks of the film suggest that the atomic layer of MoS2, even monolayer in nature definitely exists. Such results coincide with our AFM results. Hence, it is reasonable to assign them to the A1 and B1 direct excitonic transitions, respectively.[1,4,5] On the other hand, at 405 nm, the excitation is well above the A1 and B1 exciton feature, and it should be an off-resonance excitation, that is, less coupling. The red shift of the emission compared with the results in other reports can be explained by a defect trapped exciton mechanism.[4] The energy difference between the two peaks are about 50 meV under our experimental conditions, which is smaller than the reported value of MoS2 by others.[4] This smaller value means a smaller spin splitting energy in our case. Comparing the two spectra, the intensity of the spectrum in the case of right-circularly polarized light is smaller than that of left-circularly polarized light. As is well known, in monolayer MoS2, spin-orbit interaction splits the valence band. The spin projection along the c axis of the crystal is well defined and the two bands are of spin down and spin up in character. Interband transitions at the two valleys are allowed for optical excitation of opposite helicity incident along the c axis. As we do not focus the laser on the monolayer MoS2 but cover the atomic layer area, the observation of the photoexcited carrier density imbalance in different circularly polarized conditions indicates that left and right circular polarization exist in the k and k′ valleys. We know that it is dependent only on the intrinsic band parameters under the assumption of no intervalley scattering.[18] The polarization-resolved photoluminescence spectrum further confirms the presence of the atomic layer MoS2.
XPS measurements provide further information about the composition, purity and valence states of our film. XPS wide scan spectrum of the MoS2 ultrathin film in a range from 0 to 1200 eV is shown in Fig.
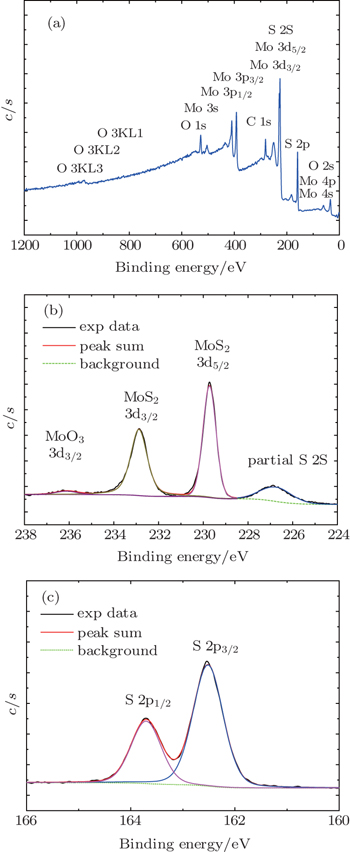 | Fig. 5. XPS spectra of the specimen with Gaussian fittings in thinner lines: (a) wide scan spectrum; (b) Mo 3d XPS spectrum; (c) S 2p XPS spectrum. |
Figure
Figure
In this work, atomic-layer MoS2 ultrathin films are synthesized using a hot filament chemical vapor deposition method. A combination of AFM, XRD, HRTEM, PL, and XPS characterization methods is used to investigate the crystal structures, valence states, and composition of the ultrathin film areas. The nucleation particles show irregular morphology, while in a larger size somewhere, the films are granular and the grains present triangle shape. The films grow with a preferred orientation (002), that is, the graphene-like MoS2 with a number of layers in the direction of the c axis perpendicular to the atomic layer.[14] The HRTEM images show the graphene-like structures of stacked layers with low density of stacking faults, and the interlayer distance of the plane is measured to be about 0.63 nm. It shows a clear quasi honeycomb-like structure and 6-fold coordination symmetry. Room-temperature PL spectra for the atomic layer MoS2 under the condition of right-circularly and left-circularly polarized light show that for both cases, there are two emission peaks around 710 nm (1.75 eV) and 686 nm (1.8 eV), which are close to the value of the direct bandgap of monolayer MoS2.Relatively high intensities of the two peaks suggest that there exists the atomic layer of MoS2, even monolayer in nature. Hence, it is reasonable to assign these two peaks to the A1 and B1 direct excitonic transitions.[1,4,5] Comparing the two spectra in both cases, the intensities of the peak A1 and B1 in the case of right-circularly polarized light is smaller than that of left-circularly polarized light, that is, valley polarization-resolved PL spectra that have been obtained. The acquirement of the polarization-resolved PL spectra further confirms the presence of the atomic layer MoS2. The XPS measurements indicate that our sample has a high purity aside from some contamination from the air. The binding energy values from the Mo 3d and S 2p core level spectra reveal that molybdenum atoms are in sulfurization state, say MoS2. The stoichiometric mole ratio of S/Mo is about 2.0–2.1 for the atomic layer MoS2. It seems that it is near the nominal value of MoS2. Furthermore, the value of the stoichiometric ratio of S/Mo also suggests that sulfur is abundant rather than deficient in our material, which coincides with the AFM result. We believe that this feature is related to the properties of the atomic layer MoS2 under our experimental conditions. The electrical measurements are carried out and the corresponding results will be published elsewhere.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 |