† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51105186).
The a-C and a-C:H films are deposited on silicon surfaces modified with and without nickel nanoparticles by using mid-frequency magnetron sputtering. The microstructures and morphologies of the films are analyzed by Raman spectroscopy and atomic force microscopy. Field emission behaviors of the deposited films with and without nickel nanoparticles modification are comparatively investigated. It is found that the hydrogen-free carbon film exhibits a high field emission current density and low turn-on electric field compared with the hydrogenated carbon film. Nickel modifying could increase the current density, whereas it has no significant effect on the turn-on electric field. The mechanism of field electron emission of a sample is discussed from the surface morphologies of the films and nickel nanoparticle roles in the interface between film and substrate.
Diamond-like carbon (DLC) films, including hydrogen-free amorphous carbon (a-C) and hydrogenated amorphous carbon (a-C:H) films, have attracted considerable attention to develop the advanced cold electron field emission materials for flat panel display and the vacuum microelectronic devices,[1] due to their negative electron affinities, low surface work functions, high thermal conductivities and outstanding chemical and physical stabilities.[2,3] However, so far the use of DLC film as a cold cathode has been restricted because of its low field emission current density. According to previous reports,[4,5] the field emission performance of DLC film was closely related to the surface morphology, composition (i.e., hydrogen content) and microstructure (i.e., sp2/sp3 bonding ratio). In recent years, great efforts have been devoted to the enhancement of the field emission properties of DLC films. Introducing the heteroatom dopant or nanoparticles into DLC film is an effective method to lower the threshold field value, increase the field current density, and enhance the field emission stability and durability.[6–10] Especially, transition metals, such as Ni and Fe were known to be very active to break and reform C–C bonds in a carbon matrix, which can strongly influence the microstructure of DLC film, thus further leading to the improvement of the field emission performance.[11,12] In these cases, the enhancement of the field emission is mainly attributed to the raised Fermi level, reduced work function, and enhanced conductivity.
The experimental and theoretical investigations have confirmed that the surface morphology is an important factor affecting the field emission properties of DLC film. The rough surface means more dense protrusions on the film surface, which could increase the field enhancement factor geometrically, thereby improving the field emission properties. Some efficient strategies have been used to produce protrusive surface structures. For example, Wei et al.[13] reported that the film surface turns from smooth to rough, becoming a peak-and-valley structure by the ion etching and bombarding method. Hart et al.[14] believed that plasma treatment can create sp2 clusters on the surface because it increased the field emission site density of the surface of DLC film.
As is well known, the surface roughness aspect of DLC film can also be controlled by the substrate roughness and can be maximized.[15,16] Therefore, in this work we prepare the a-C and a-C:H films by nickel nanoparticles modifying silicon substrate to regulate surface morphology using mid-frequency magnetron sputtering. The influence of nickel nanoparticle modification on the field emission property of the DLC film is comparatively investigated. To the best of our knowledge, there is no report on the field emission of DLC film deposited on Ni nanoparticles modified silicon substrate. The structures and surface morphologies of the films are investigated to clarify their field emission performances.
The n-Si (100) substrates were ultrasonically cleaned in alcohol and acetone. Ni nanoparticles (about 30 nm–80 nm in size) were ultrasonically dispersed in alcohol, and then dropped on the Si substrate surface. The silicon substrate modified with Ni nanoparticles was dried overnight in the atmospheric environment. Subsequently, the a-C films of approximately 180 nm in thickness were deposited on the pre-treated substrates by using mid-frequency magnetron sputtering graphite targets under 120-sccm Ar flow rate. The a-C:H films of approximately 1.2 μm in thickness were deposited by using mid-frequency magnetron sputtering graphite targets under a mixture atmosphere of 120-sccm Ar and 6-sccm CH4 flow rate. The background pressure was 3.9×10−3 Pa. The middle frequency (20 kHz) magnetron sputtering targets were operated at a constant current of 2.2 A with discharge voltages in ranges of 670 V–760 V for a-C films and 390 V–480 V for a-C:H films respectively. The plus bias voltage applied to the substrate was −50 V with 4-kHz frequency and 80% duty cycle. The deposition time was 90 min for all the films.
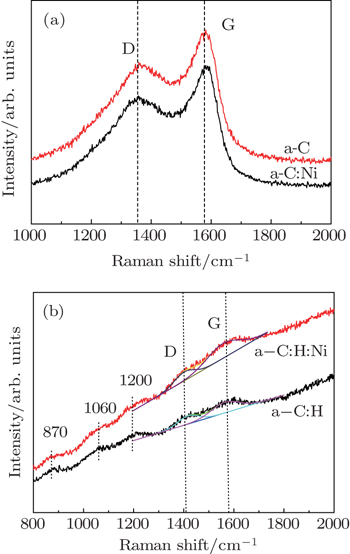
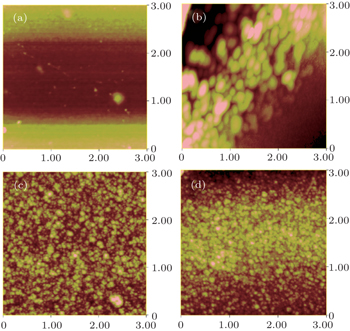
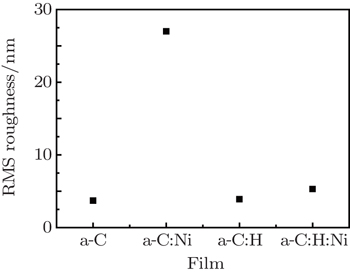
The surface morphologies and root-mean-square (RMS) surface roughness values of the films were acquired in tapping mode using an SPM (Nano IIIa) atomic force microscope (AFM). Raman spectra were recorded by a Horiba Jobin Yvon HR800 Raman spectrometer, with a 532-nm Ar ion laser used as an excitation resource. Field emission experiments were performed in a vacuum chamber with a pressure of 1.0×10−6 Pa. The measurements were conducted on a standard parallel-plate-electrode configuration where a stainless-steel plate was used as an anode current collector, and the film fixed onto a copper stage with conductive tape served as a cathode.[17] The distance between the film surface and the anode was adjusted to about 300 μm by a spiral micrometer prior to the measurements. The voltage was applied from 300 V to 3600 V in steps of 30 V. The anode and the cathode were connected by a computer-controlled Keithley 248 source meter to record the corresponding current.
Figure
Figure
Figure
Figure
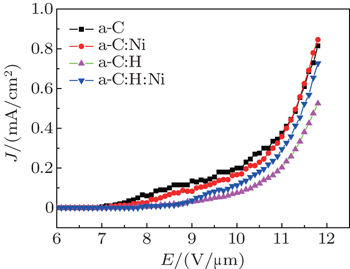
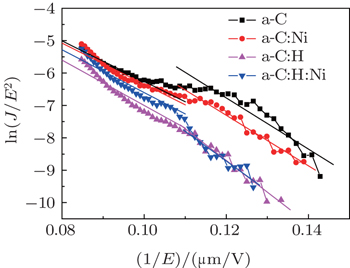
The Fowler–Nordheim (FN) plots of the films, i.e., plots of ln (J/E2) versus 1/E, are obtained in Fig.
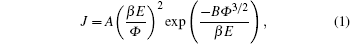
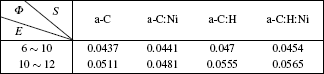
| Table 1. Work functions of the films at low and high electric field parts. The parameters Φ in unit eV and E in units V·μm−1. . |
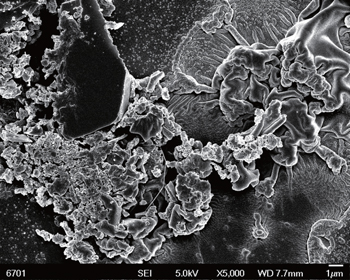
Moreover, besides surface roughening, the role of nickel nanoparticles in transport of electrons is also important, which should be considered in this case. On the one hand, it is reasonable to believe that the nickel modification can offer the higher conductivity between the film and silicon substrate, which is beneficial to transport of field electrons. However, on the other hand, the nickel modification reduces the adhesion between film and silicon substrate, leading to the wrinkles, cracks and even partly peeling off from the substrate as shown in Fig.
In this paper, we report the field-emission properties of the a-C and a-C:H films deposited on silicon surfaces modified with and without nickel nanoparticles by mid-frequency magnetron sputtering. It is shown that the hydrogen-free DLC film exhibits the higher field emission current density and lower turn-on electric field than the hydrogenated DLC film. The Ni nanoparticle modification can improve the field-emission performance of the DLC film. Especially, the field emission current density slightly increases from 815 μA/cm2 to 846 μA/cm2 for the hydrogen-free DLC film, whereas it markedly increases from 525 μA/cm2 to 727 μA/cm2 for the hydrogenated DLC film. There are no distinct changes in the turn-on electric field of the film before and after modification.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 |