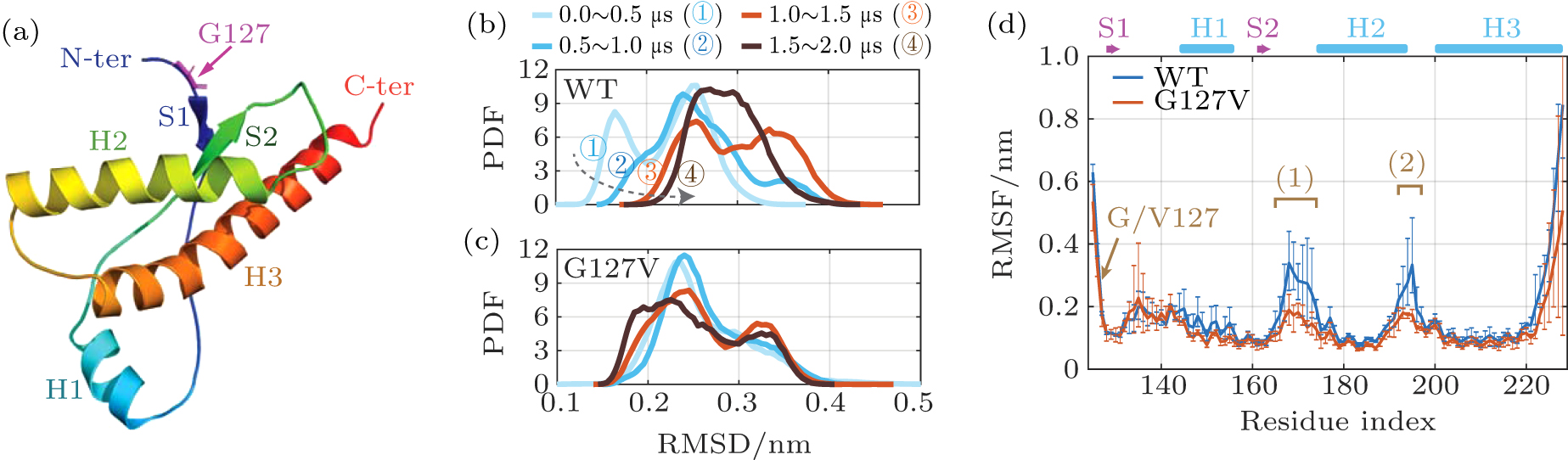
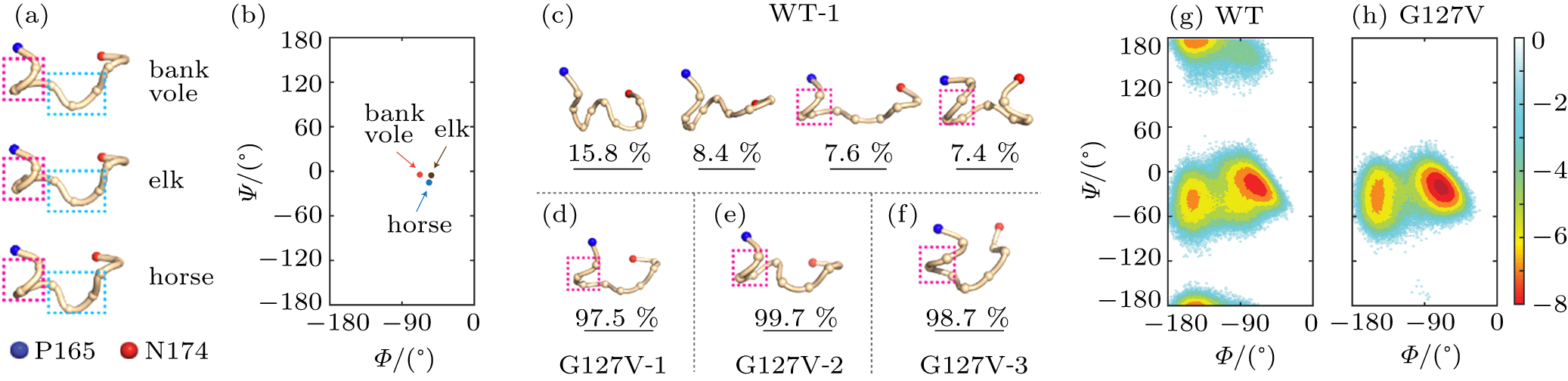
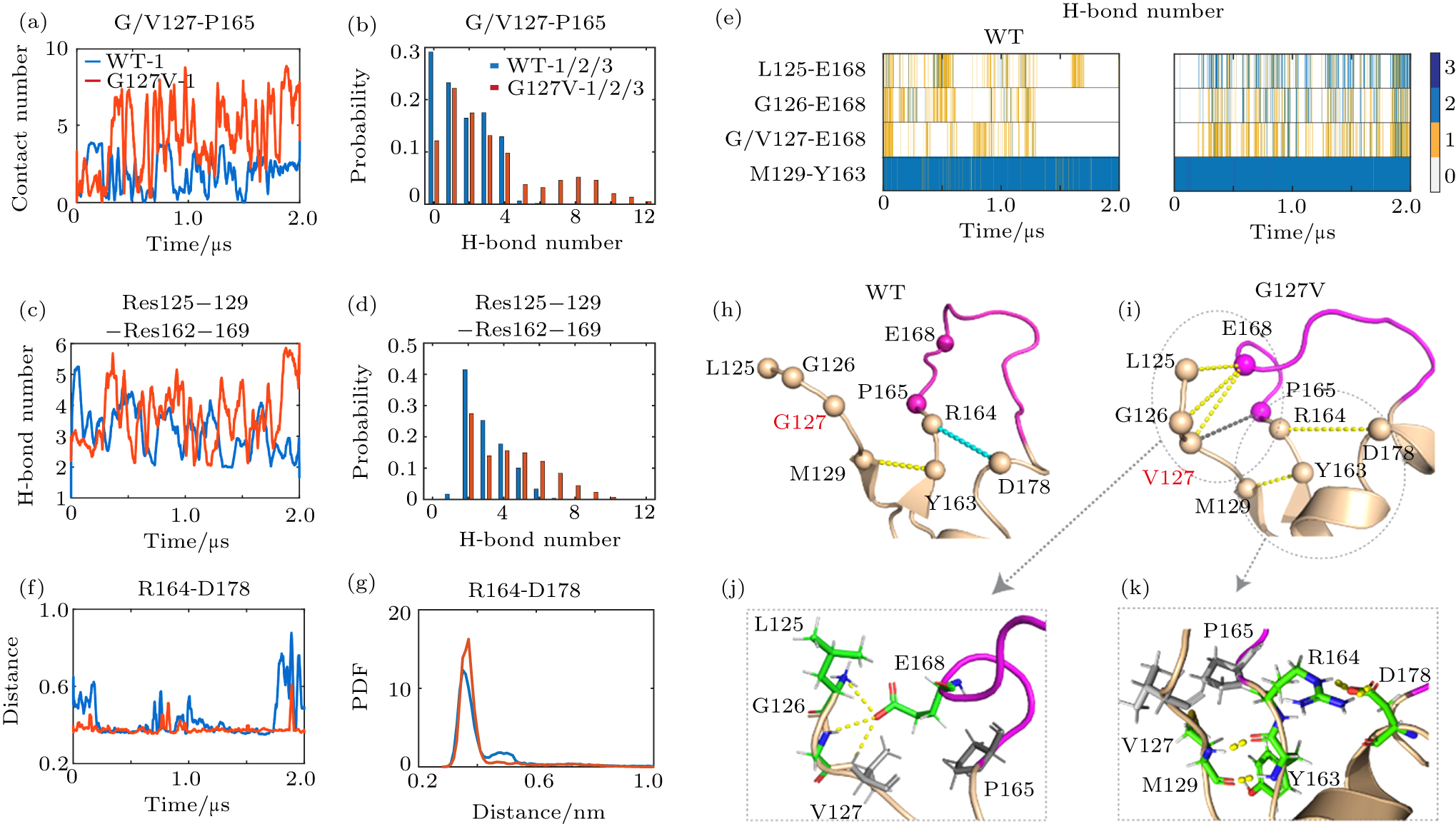
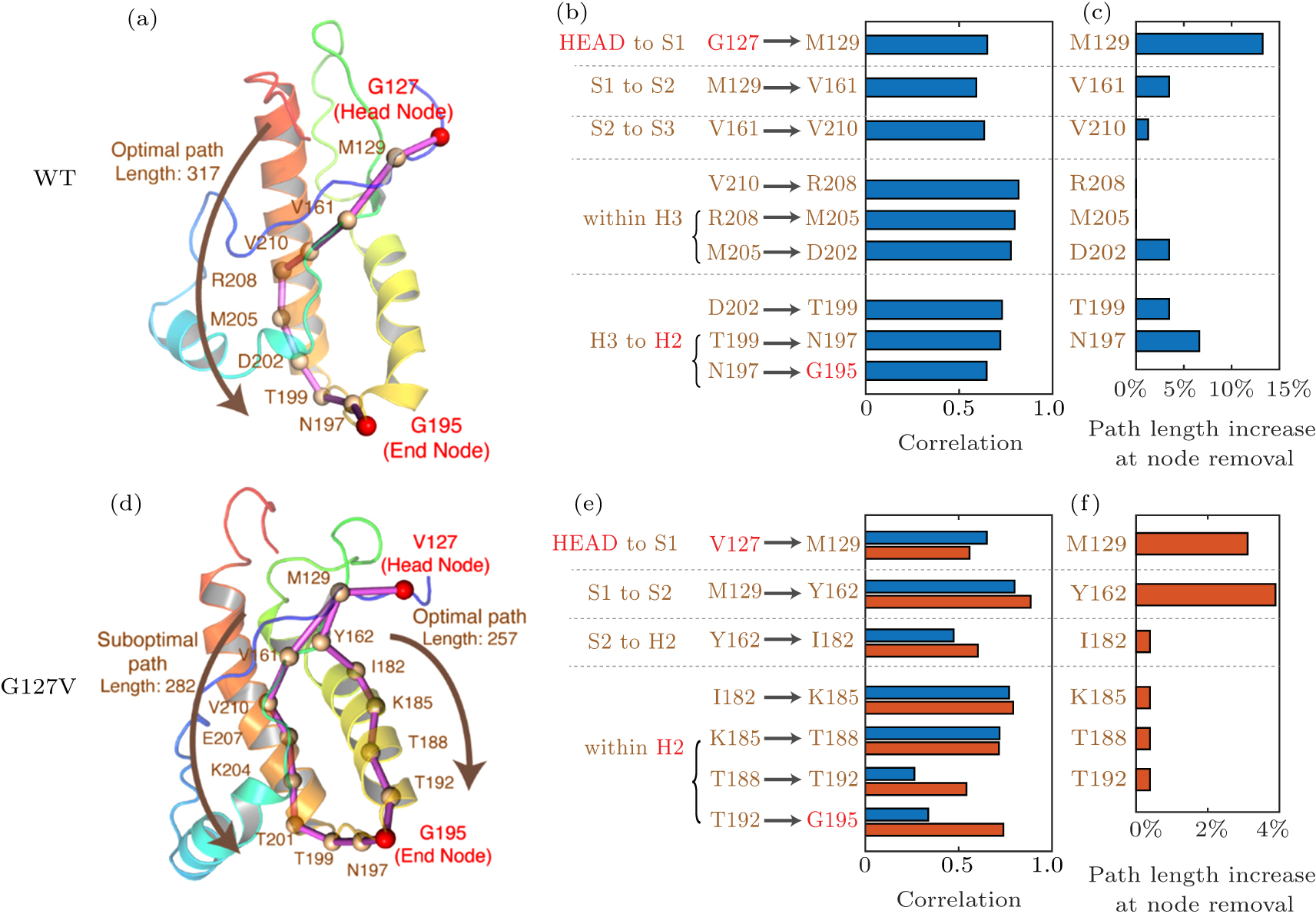
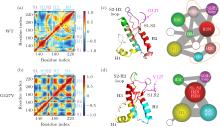
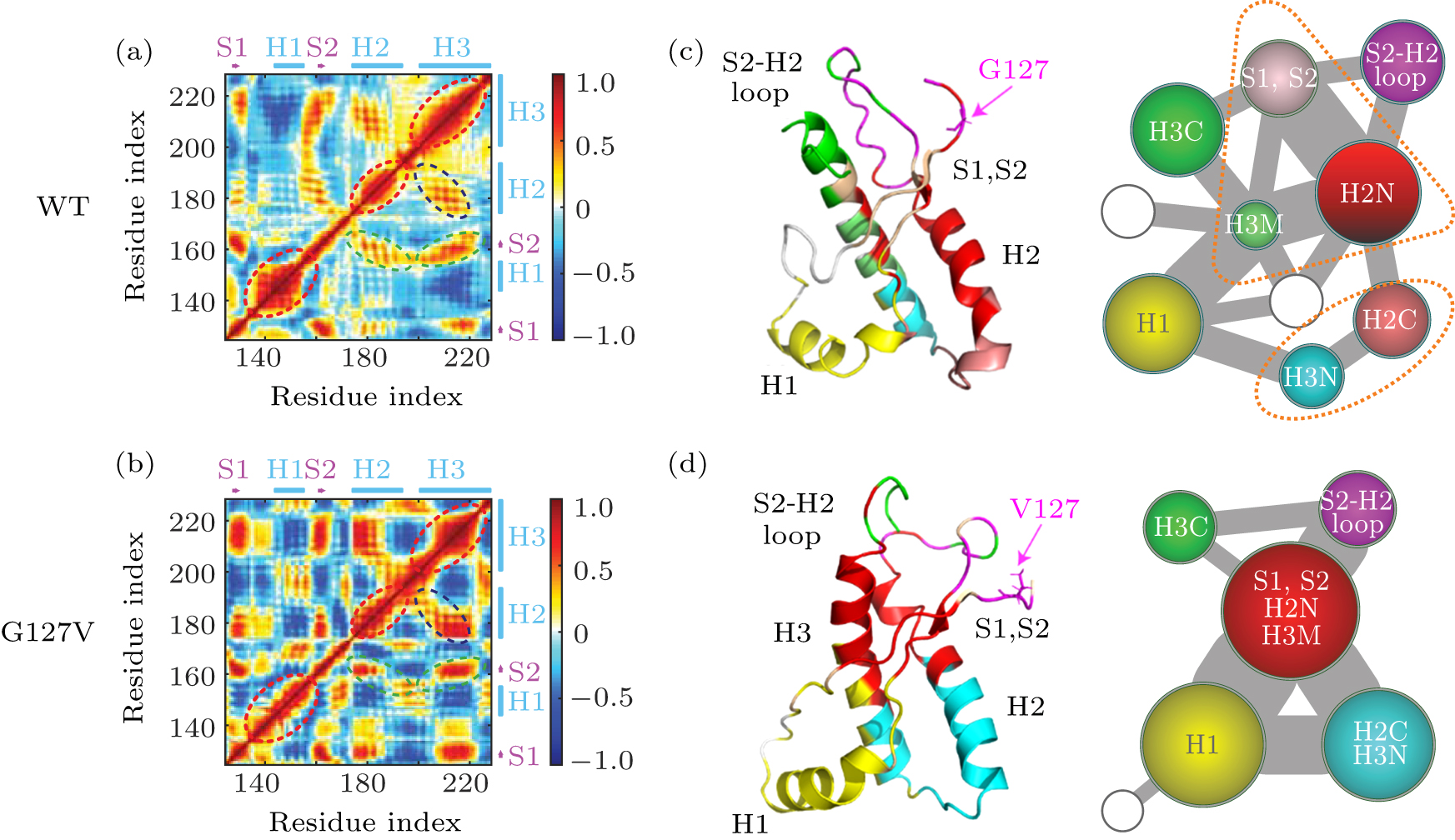
中国物理B ›› 2020, Vol. 29 ›› Issue (10): 108710-.doi: 10.1088/1674-1056/aba9ba
Yiming Tang(唐一鸣)1, Yifei Yao(姚逸飞)1, Guanghong Wei(韦广红)1,†( )
)
-
收稿日期:2020-06-24修回日期:2020-07-19接受日期:2020-07-28出版日期:2020-10-05发布日期:2020-10-05 -
通讯作者:Guanghong Wei(韦广红)
Structural and dynamical mechanisms of a naturally occurring variant of the human prion protein in preventing prion conversion
Yiming Tang(唐一鸣), Yifei Yao(姚逸飞), and Guanghong Wei(韦广红)†
- 1
Department of Physics, State Key Laboratory of Surface Physics and Key Laboratory for Computational Physical Science (Ministry of Education), and Multiscale Research Institute of Complex Systems, Fudan University , Shanghai 200433,China
-
Received:2020-06-24Revised:2020-07-19Accepted:2020-07-28Online:2020-10-05Published:2020-10-05 -
Contact:†Corresponding author. E-mail:ghwei@fudan.edu.cn -
About author:†Corresponding author. E-mail: ghwei@fudan.edu.cn* Project supported by the Key Program of the National Key Research and Development Program of China (Grant No. 2016YFA0501702) and the National Natural Science Foundation of China (Grant No. 11674065).
中图分类号: (Biomolecules: structure and physical properties)
- 87.15.-v
引用本文
Yiming Tang(唐一鸣), Yifei Yao(姚逸飞), Guanghong Wei(韦广红). [J]. 中国物理B, 2020, 29(10): 108710-.
Yiming Tang(唐一鸣), Yifei Yao(姚逸飞), and Guanghong Wei(韦广红)†. Structural and dynamical mechanisms of a naturally occurring variant of the human prion protein in preventing prion conversion[J]. Chin. Phys. B, 2020, 29(10): 108710-.
| [1] |
GREIG J R 1950 J. Comp. Pathol. 60 263 DOI: 10.1016/S0368-1742(50)80024-3
|
| [2] |
Hunter N 2003 Br. Med. Bull. 66 171 DOI: 10.1093/bmb/66.1.171
|
| [3] |
Williams E S, Young S 1980 J. Wildl. Dis. 16 89 DOI: 10.7589/0090-3558-16.1.89
|
| [4] |
Chatigny M A, Prusiner S B 1980 Rev. Infect. Dis. 2 713 DOI: 10.1093/clinids/2.5.713
|
| [5] |
Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofmar A, Smith P G 1996 Lancet 347 921 DOI: 10.1016/S0140-6736(96)91412-9
|
| [6] |
Collinge J 1999 Lancet 354 317 DOI: 10.1016/S0140-6736(99)05128-4
|
| [7] |
Lugaresi E, Medori R, Montagna P, Baruzzi A, Cortelli P, Lugaresi A, Tinuper P, Zucconi M, Gambetti P 1986 N. Engl. J. Med. 315 997 DOI: 10.1056/NEJM198610163151605
|
| [8] |
Medori R, Tritschler H-J, LeBlanc A, Villare F, Manetto V, Chen H Y, Xue R, Leal S, Montagna P, Cortelli P, Tinuper P, Avoni P, Mochi M, Baruzzi A, Hauw J J, Ott J, Lugaresi E, Autilio-Gambetti L, Gambetti P 1992 N. Engl. J. Med. 326 444 DOI: 10.1056/NEJM199202133260704
|
| [9] |
Gajdusek D C 1963 Trans. R. Soc. Trop. Med. Hyg. 57 151 DOI: 10.1016/0035-9203(63)90057-9
|
| [10] |
Matthews J D, Glasse R, Lindenbaum S 1968 Lancet 2 449
|
| [11] |
Griffith J S 1967 Nature 215 1043 DOI: 10.1038/2151043a0
|
| [12] |
Farquhar C F, Somerville R A, Bruce M E 1998 Nature 391 345 DOI: 10.1038/34818
|
| [13] |
Zou W Q, Gambetti P 2005 Cell 121 155 DOI: 10.1016/j.cell.2005.04.002
|
| [14] |
Soto C 2011 Trends Biochem. Sci. 36 151 DOI: 10.1016/j.tibs.2010.11.001
|
| [15] |
Prusiner S B 1982 Science 216 136 DOI: 10.1126/science.6801762
|
| [16] |
Tuite M F, Serio T R 2010 Nat. Rev. Mol. Cell Biol. 11 823 DOI: 10.1038/nrm3007
|
| [17] |
Frost B, Diamond M I 2010 Nat. Rev. Neurosci. 11 155 DOI: 10.1038/nrn2786
|
| [18] |
Nussbaum J M, Schilling S, Cynis H, Silva A, Swanson E, Wangsanut T, Tayler K, Wiltgen B, Hatami A, Rönicke R, Reymann K, Hutter-Paier B, Alexandru A, Jagla W, Graubner S, Glabe C G, Demuth H U, Bloom G S 2012 Nature 485 651 DOI: 10.1038/nature11060
|
| [19] |
Chaari A, Ladjimi M 2019 Int. J. Biol. Macromol. 136 57 DOI: 10.1016/j.ijbiomac.2019.06.050
|
| [20] |
Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann D M A, Hasegawa M 2013 Brain 136 1128 DOI: 10.1093/brain/awt037
|
| [21] |
Chu Y, Kordower J H 2015 Curr. Neurol. Neurosci. Rep. 15 1 DOI: 10.1007/s11910-014-0514-0
|
| [22] |
Polymenidou M, Cleveland D W 2011 Cell 147 498 DOI: 10.1016/j.cell.2011.10.011
|
| [23] |
Prasad A, Bharathi V, Sivalingam V, Girdhar A, Patel B K 2019 Front. Mol. Neurosci. 12 25 DOI: 10.3389/fnmol.2019.00025
|
| [24] |
Abid K, Soto C 2006 Cell. Mol. Life Sci. 63 2342 DOI: 10.1007/s00018-006-6140-5
|
| [25] |
Riesner D 2003 Br. Med. Bull. 66 21 DOI: 10.1093/bmb/66.1.21
|
| [26] |
Kupfer L, Hinrichs W, Groschup M 2009 Curr. Mol. Med. 9 826 DOI: 10.2174/156652409789105543
|
| [27] |
Calzolai L, Zahn R 2003 J. Biol. Chem. 278 35592 DOI: 10.1074/jbc.M303005200
|
| [28] |
Zahn R, Liu A, Lührs T, Riek R, Von Schroetter C, Garcia F L, Billeter M, Calzolai L, Wider G, Wüthrich K 2000 Proc. Natl. Acad. Sci. USA 97 145 DOI: 10.1073/pnas.97.1.145
|
| [29] |
Calzolai L, Lysek D A, Güntert P, Von Schroetter C, Riek R, Zahn R, Wüthrich K 2000 Proc. Natl. Acad. Sci. USA 97 8340 DOI: 10.1073/pnas.97.15.8340
|
| [30] |
Knaus K J, Morillas M, Swietnicki W, Malone M, Surewicz W K, Yee V C 2001 Nat. Struct. Biol. 8 770 DOI: 10.1038/nsb0901-770
|
| [31] |
Westergard L, Christensen H M, Harris D A 2007 Biochim. Biophys. Acta. (BBA)-Mol. Basis Dis. 1772 629 DOI: 10.1016/j.bbadis.2007.02.011
|
| [32] |
Provenzano L, Ryan Y, Hilton D A, Lyons-Rimmer J, Dave F, Maze E A, Adams C L, Rigby-Jones R, Ammoun S, Hanemann C O 2017 Oncogene 36 6132 DOI: 10.1038/onc.2017.200
|
| [33] |
Linsenmeier L, Altmeppen H C, Wetzel S, Mohammadi B, Saftig P, Glatzel M 2017 Biochim. Biophys. Acta-Mol. Cel. Res. 1864 2128 DOI: 10.1016/j.bbamcr.2017.06.022
|
| [34] |
Wu G R, Mu T C, Gao Z X, Wang J, Sy M S, Li C Y 2017 J. Biol. Chem. 292 18747 DOI: 10.1074/jbc.M117.787283
|
| [35] |
Linden R 2017 Front. Mol. Neurosci. 10 77 DOI: 10.3389/fnmol.2017.00077
|
| [36] |
Franzmann T M, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse A S, Nüske E, Richter D, Baumeister W, Grill S W, Pappu R V, Hyman A A, Alberti S 2018 Science 359 6371 DOI: 10.1126/science.aao5654
|
| [37] |
Pan K M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B 1993 Proc. Natl. Acad. Sci. USA 90 10962 DOI: 10.1073/pnas.90.23.10962
|
| [38] |
Diaz-Espinoza R, Soto C 2012 Nat. Struct. Mol. Biol. 19 370 DOI: 10.1038/nsmb.2266
|
| [39] |
Wille H, Requena J 2018 Pathogens 7 20 DOI: 10.3390/pathogens7010020
|
| [40] |
Zou W-Q, Zhou X, Yuan J, Xiao X 2011 Prion 5 172 DOI: 10.4161/pri.5.3.16894
|
| [41] |
Ma J, Lindquist S 2002 Science 298 1785 DOI: 10.1126/science.1073619
|
| [42] |
Jackson G S 1999 Science 283 1935 DOI: 10.1126/science.283.5409.1935
|
| [43] |
Wang H, Rhoads D D, Appleby B S 2019 Curr. Opin. Infect. Dis. 32 272 DOI: 10.1097/QCO.0000000000000552
|
| [44] |
Petersen R B, Parchi P, Richardson S L, Urig C B, Gambetti P 1996 J. Biol. Chem. 271 12661 DOI: 10.1074/jbc.271.21.12661
|
| [45] |
Zarranz J J, Digon A, Atarés B, Rodríguez-Martinez A B, Arce A, Carrera N, Fernández-Manchola I, Fernández-Martínez M, Fernández-Maiztegui C, Forcadas I, Galdos L, Gómez-Esteban J C, Ibáñez A, Lezcano E, De López Munain A, Martí-Massó J F, Mendibe M M, Urtasun M, Uterga J M, Saracibar N, Velasco F, De Pancorbo M M 2005 J. Neurol. Neurosurg. Psychiatry 76 1491 DOI: 10.1136/jnnp.2004.056606
|
| [46] |
Woulfe J, Kertesz A, Frohn I, Bauer S, George-Hyslop P S, Bergeron C 2005 Acta Neuropathol. 110 317 DOI: 10.1007/s00401-005-1054-0
|
| [47] |
Tartaglia M C, Thai J N, See T, Kuo A, Harbaugh R, Raudabaugh B, Cali I, Sattavat M, Sanchez H, DeArmond S J, Geschwind M D 2010 J. Neuropathol. Exp. Neurol. 69 1220 DOI: 10.1097/NEN.0b013e3181ffc39c
|
| [48] |
Palmer M S, Dryden A J, Hughes J T, Collinge J 1991 Nature 352 340 DOI: 10.1038/352340a0
|
| [49] |
Shibuya S, Higuchi J, Shin R W, Tateishi J, Kitamoto T 1998 Ann. Neurol. 43 826 DOI: 10.1002/ana.v43:6
|
| [50] |
Soldevila M, Calafell F, Andrés A M, Yagüe J, Helgason A, Stefánsson K, Bertranpetit J 2003 Hum. Mutat. 22 104 DOI: 10.1126/science.aao5654
|
| [51] |
Takayanagi M, Suzuki K, Nakamura T, Hirata K, Satoh K, Kitamoto T 2018 Rinsho Shinkeigaku 58 682 DOI: 10.5692/clinicalneurol.cn-001206
|
| [52] |
Mead S, Whitfield J, Poulter M, Shah P, Uphill J, Campbell T, Al-Dujaily H, Hummerich H, Beck J, Mein C A, Verzilli C, Whittaker J, Alpers M P, Collinge J 2009 N. Engl. J. Med. 361 2056 DOI: 10.1056/NEJMoa0809716
|
| [53] |
Asante E A, Smidak M, Grimshaw A, Houghton R, Tomlinson A, Jeelani A, Jakubcova T, Hamdan S, Richard-Londt A, Linehan J M, Brandner S, Alpers M, Whitfield J, Mead S, Wadsworth J D F, Collinge J 2015 Nature 522 478 DOI: 10.1038/nature14510
|
| [54] |
Sabareesan A T, Udgaonkar J B 2017 Biochemistry 56 5931 DOI: 10.1021/acs.biochem.7b00894
|
| [55] |
Pan A C, Jacobson D, Yatsenko K, Sritharan D, Weinreich T M, Shaw D E 2019 Proc. Natl. Acad. Sci. USA 116 4244 DOI: 10.1073/pnas.1815431116
|
| [56] |
Bermudez M, Mortier J, Rakers C, Sydow D, Wolber G 2016 Drug Discov. Today 21 1799 DOI: 10.1016/j.drudis.2016.07.001
|
| [57] |
Plattner N, Doerr S, De Fabritiis G, Noé F 2017 Nat. Chem. 9 1005 DOI: 10.1038/nchem.2785
|
| [58] |
Zhou S, Shi D, Liu X, Liu H, Yao X 2016 Sci. Rep. 6 24765 DOI: 10.1038/srep24765
|
| [59] |
Zheng Z, Zhang M, Wang Y, Ma R, Guo C, Feng L, Wu J, Yao H, Lin D 2018 Sci. Rep. 8 1 DOI: 10.1038/s41598-017-17765-5
|
| [60] |
El-Bastawissy E, Knaggs M H, Gilbert I H 2001 J. Mol. Graph. Model. 20 145 DOI: 10.1016/S1093-3263(01)00113-9
|
| [61] |
Sekijima M, Motono C, Yamasaki S, Kaneko K, Akiyama Y 2003 Biophys. J. 85 1176 DOI: 10.1016/S0006-3495(03)74553-6
|
| [62] |
Mandujano-Rosas L A, Osorio-González D, Reyes-Romero P G, Mulia-Rodríguez J 2014 Open J. Biophys. 04 169 DOI: 10.4236/ojbiphy.2014.44016
|
| [63] |
Borgohain G, Dan N, Paul S 2016 Biophys. Chem. 213 32 DOI: 10.1016/j.bpc.2016.03.004
|
| [64] |
Gao Y, Zhu T, Zhang C, Zhang J Z H, Mei Y 2018 Chem. Phys. Lett. 706 594 DOI: 10.1016/j.cplett.2018.07.014
|
| [65] |
Barducci A, Chelli R, Procacci P, Schettino V, Gervasio F L, Parrinello M 2006 J. Am. Chem. Soc. 128 2705 DOI: 10.1021/ja057076l
|
| [66] |
Caldarulo E, Barducci A, Wüthrich K, Parrinello M 2017 Proc. Natl. Acad. Sci. USA 114 9617 DOI: 10.1073/pnas.1712155114
|
| [67] |
Huang D, Caflisch A 2015 J. Am. Chem. Soc. 137 2948 DOI: 10.1021/ja511568m
|
| [68] |
Avbelj M, Hafner-Bratkovič I, Jerala R 2011 Biochem. Biophys. Res. Commun. 413 521 DOI: 10.1016/j.bbrc.2011.08.125
|
| [69] |
Kurt T D, Aguilar-Calvo P, Jiang L, Rodriguez J A, Alderson N, Eisenberg D S, Sigurdson C J 2017 J. Biol. Chem. 292 19076 DOI: 10.1074/jbc.M117.794107
|
| [70] |
Sigurdson C J, Nilsson K P R, Hornemann S, Manco G, Fernández-Borges N, Schwarz P, Castilla J, Wüthrich K, Aguzzi A 2010 J. Clin. Invest. 120 2590 DOI: 10.1172/JCI42051
|
| [71] |
Christen B, Pérez D R, Hornemann S, Wüthrich K 2008 J. Mol. Biol. 383 306 DOI: 10.1016/j.jmb.2008.08.045
|
| [72] |
Gossert A D, Bonjour S, Lysek D A, Fiorito F, Wüthrich K 2005 Proc. Natl. Acad. Sci. USA 102 646 DOI: 10.1073/pnas.0409008102
|
| [73] |
Sweeting B, Brown E, Khan M Q, Chakrabartty A, Pai E F 2013 PLoS One 8 e63047 DOI: 10.1371/journal.pone.0063047
|
| [74] |
Pérez D R, Damberger F F, Wüthrich K 2010 J. Mol. Biol. 400 121 DOI: 10.1016/j.jmb.2010.04.066
|
| [75] |
Agarwal S, Döring K, Gierusz L A, Iyer P, Lane F M, Graham J F, Goldmann W, Pinheiro T J T, Gill A C 2015 Sci. Rep. 5 15528 DOI: 10.1038/srep15528
|
| [76] |
Kaneko K, Zulianello L, Scott M, Cooper C M, Wallace A C, James T L, Cohen F E, Prusiner S B 1997 Proc. Natl. Acad. Sci. USA 94 10069 DOI: 10.1073/pnas.94.19.10069
|
| [77] |
Knaus K J, Morillas M, Swietnicki W, Malone M, Surewicz W K, Yee V C 2001 Nat. Struct. Biol. 8 770 DOI: 10.1038/nsb0901-770
|
| [78] |
Bjorndahl T C, Zhou G P, Liu X, Perez-Pineiro R, Semenchenko V, Saleem F, Acharya S, Bujold A, Sobsey C A, Wishart D S 2011 Biochemistry 50 1162 DOI: 10.1021/bi101435c
|
| [79] |
Singh J, Kumar H, Sabareesan A T, Udgaonkar J B 2014 J. Am. Chem. Soc. 136 16704 DOI: 10.1021/ja510964t
|
| [80] |
Gower J C, Ross G J S 1969 Appl. Stat. 18 54 DOI: 10.2307/2346439
|
| [81] |
Huang J J, Li X N, Liu W L, Yuan H Y, Gao Y, Wang K, Tang B, Pang D W, Chen J, Liang Y 2020 J. Mol. Biol. 432 828 DOI: 10.1016/j.jmb.2019.11.020
|
| [82] |
Zuegg J, Gready J E 1999 Biochemistry 38 13862 DOI: 10.1021/bi991469d
|
| [83] |
Zhang J 2011 J. Theor. Biol. 269 88 DOI: 10.1016/j.jtbi.2010.10.020
|
| [84] |
Miao Y, Nichols S E, Gasper P M, Metzger V T, McCammon J A 2013 Proc. Natl. Acad. Sci. USA 110 10982 DOI: 10.1073/pnas.1309755110
|
| [85] |
del Sol A, Tsai C J, Ma B, Nussinov R 2009 Structure 17 1042 DOI: 10.1016/j.str.2009.06.008
|
| [86] |
Csermely P, Palotai R, Nussinov R 2010 Nat. Prec. 1 1 DOI: 10.1038/npre.2010.4422.1
|
| [87] |
Csermely P, Korcsmáros T, Kiss H J M, London G, Nussinov R 2013 Pharmacol. Ther. 138 333 DOI: 10.1016/j.pharmthera.2013.01.016
|
| [88] |
Sethi A, Eargle J, Black A A, Luthey-Schulten Z 2009 Proc. Natl. Acad. Sci. 106 6620 DOI: 10.1073/pnas.0810961106
|
| [89] |
Koehorst J J, van Dam J C J, Saccenti E, Martins V A P, Suarez-Diez M, Schaap P J 2018 Bioinformatics 34 1401 DOI: 10.1093/bioinformatics/btx767
|
| [90] |
Ahalawat N, Murarka R K 2015 J. Biomol. Struct. Dyn. 33 2192 DOI: 10.1080/07391102.2014.996609
|
| [91] |
Yang J, Liu H, Liu X, Gu C, Luo R, Chen H F 2016 J. Chem. Inf. Model. 56 1184 DOI: 10.1021/acs.jcim.6b00115
|
| [92] |
Alcalá-Corona S A, Velázquez-Caldelas T E, Espinal-Enríquez J, Hernández-Lemus E 2016 Front. Physiol. 7 184 DOI: 10.3389/fphys.2016.00184
|
| [93] |
Guo C, Zhou H X 2016 Proc. Natl. Acad. Sci. USA 113 E6776
|
| [94] |
Girvan M, Newman M E J 2002 Proc. Natl. Acad. Sci. USA 99 7821 DOI: 10.1073/pnas.122653799
|
| [95] |
The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC
|
| [96] |
Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis J L, Dror R O, Shaw D E 2010 Proteins Struct. Funct. Bioinforma. 78 1950 DOI: 10.1002/prot.22711
|
| [97] |
Abraham M J, Murtola T, Schulz R, Páll S, Smith J C, Hess B, Lindah E 2015 SoftwareX 1 19 DOI: 10.1016/j.softx.2015.06.001
|
| [98] |
Darden T, York D, Pedersen L 1993 J. Chem. Phys. 98 10089 DOI: 10.1063/1.464397
|
| [99] |
Bussi G, Donadio D, Parrinello M 2007 J. Chem. Phys. 126 014101 DOI: 10.1063/1.2408420
|
| [100] |
Parrinello M, Rahman A 1981 J. Appl. Phys. 52 7182 DOI: 10.1063/1.328693
|
| [101] |
Páll S, Hess B 2013 Comput. Phys. Commun. 184 2641 DOI: 10.1016/j.cpc.2013.06.003
|
| [102] |
Papadimitriou C, Sideri M 1999 J. Log. Program. 41 129 DOI: 10.1016/S0743-1066(99)00013-8
|
| [103] |
Newman M E J, Girvan M 2004 Phys. Rev. E. 69 026113 DOI: 10.1103/PhysRevE.69.026113
|
| [104] |
Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graph. 14 33 DOI: 10.1016/0263-7855(96)00018-5
|
| [1] | Cong-Min Ji(祭聪敏), Yusong Tu(涂育松), and Yuan-Yan Wu(吴园燕). Heterogeneous hydration patterns of G-quadruplex DNA[J]. 中国物理B, 2023, 32(2): 28702-028702. |
| [2] | Deng-Ke Xi(席登科), Xian-Hui Zhang(张先徽), Si-Ze Yang(杨思泽), Seong Shan Yap(叶尚姗), Kenji Ishikawa(石川健治), Masura Hori (堀勝), and Seong Ling Yap(叶尚凌). Impact of microsecond-pulsed plasma-activated water on papaya seed germination and seedling growth[J]. 中国物理B, 2022, 31(12): 128201-128201. |
| [3] | Jing Wang(王静), Hua Li(李华), Xiankai Jiang(姜先凯), Bin Wu(吴斌), Jun Guo(郭俊), Xiurong Su(苏秀榕), Xingfei Zhou(周星飞), Yu Wang(王宇), Geng Wang(王耿), Heping Geng(耿和平), Zheng Jiang(姜政), Fang Huang(黄方), Gang Chen(陈刚), Chunlei Wang(王春雷), Haiping Fang(方海平), and Chenqi Xu(许琛琦). Peptide backbone-copper ring structure: A molecular insight into copper-induced amyloid toxicity[J]. 中国物理B, 2022, 31(10): 108702-108702. |
| [4] | Jingjing Xue(薛晶晶), Xinpeng Li(李新朋), Rongri Tan(谈荣日), and Wenjun Zong(宗文军). Molecular dynamics simulations of A-DNA in bivalent metal ions salt solution[J]. 中国物理B, 2022, 31(4): 48702-048702. |
| [5] | Guoyuan Qi(齐国元) and Zimou Wang(王子谋). Modeling and dynamics of double Hindmarsh-Rose neuron with memristor-based magnetic coupling and time delay[J]. 中国物理B, 2021, 30(12): 120516-120516. |
| [6] | Tiantian Yang(杨甜甜), Tianxiang Yu(俞天翔), Wenhui Zhao(赵文辉), and Dongdong Lin(林冬冬). Tunable inhibition of β-amyloid peptides by fast green molecules[J]. 中国物理B, 2021, 30(8): 88701-088701. |
| [7] | . [J]. 中国物理B, 2021, 30(1): 18702-. |
| [8] | 封国宝, 刘璐, 崔万照, 王芳. Electron beam irradiation on novel coronavirus (COVID-19): A Monte-Carlo simulation[J]. 中国物理B, 2020, 29(4): 48703-048703. |
| [9] | 周旭聪, 石尚, 李飞, 杨玉军, 陈京, 孟庆田, 王兵兵. Asymmetric structure of atomic above-threshold ionization spectrum in two-color elliptically polarized laser fields[J]. 中国物理B, 2019, 28(10): 103201-103201. |
| [10] | 石尚, 金发成, 王兵兵. Angle-resolved spectra of the direct above-threshold ionization of diatomic molecule in IR+XUV laser fields[J]. 中国物理B, 2019, 28(2): 23202-023202. |
| [11] | 童鑫, 胡蕊, 李晓晴, 赵清. Probing conformational change of T7 RNA polymerase and DNA complex by solid-state nanopores[J]. 中国物理B, 2018, 27(11): 118705-118705. |
| [12] | 杨志伟, 郝东晓, 车一卓, 杨嘉辉, 张磊, 张胜利. Mutation-induced spatial differences in neuraminidase structure and sensitivity to neuraminidase inhibitors[J]. 中国物理B, 2018, 27(1): 18704-018704. |
| [13] | V N Likhachev, O I Shevaleevskii, G A Vinogradov. Quantum dynamics of charge transfer on the one-dimensional lattice: Wave packet spreading and recurrence[J]. 中国物理B, 2016, 25(1): 18708-018708. |
| [14] | 蒋杨伟, 冉诗勇, 何林李, 王向红, 章林溪. Decondensation behavior of DNA chains induced by multivalent cations at high salt concentrations: Molecular dynamics simulations and experiments[J]. 中国物理B, 2015, 24(11): 118701-118701. |
| [15] | Slobodan Zeković, Annamalai Muniyappan, Slobodan Zdravković, Louis Kavitha. Employment of Jacobian elliptic functions for solving problems in nonlinear dynamics of microtubules[J]. 中国物理B, 2014, 23(2): 20504-020504. |
|
||