† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 51602340, 51702355, and 61674167), the Natural Science Foundation of Beijing Municipality of China (Grant No. 4192064), the National Key Research Program of China (Grant Nos. 2018YFB1500500 and 2018YFB1500200), and the JKW Project of China (Grant No. 31512060106).
Based on the surface passivation of n-type silicon in a silicon drift detector (SDD), we propose a new passivation structure of SiO2/Al2O3/SiO2 passivation stacks. Since the SiO2 formed by the nitric-acid-oxidation-of-silicon (NAOS) method has good compactness and simple process, the first layer film is formed by the NAOS method. The Al2O3 film is also introduced into the passivation stacks owing to exceptional advantages such as good interface characteristic and simple process. In addition, for requirements of thickness and deposition temperature, the third layer of the SiO2 film is deposited by plasma enhanced chemical vapor deposition (PECVD). The deposition of the SiO2 film by PECVD is a low-temperature process and has a high deposition rate, which causes little damage to the device and makes the SiO2 film very suitable for serving as the third passivation layer. The passivation approach of stacks can saturate dangling bonds at the interface between stacks and the silicon substrate, and provide positive charge to optimize the field passivation of the n-type substrate. The passivation method ultimately achieves a good combination of chemical and field passivations. Experimental results show that with the passivation structure of SiO2/Al2O3/SiO2, the final minority carrier lifetime reaches 5223 μs at injection of 5×1015 cm−3. When it is applied to the passivation of SDD, the leakage current is reduced to the order of nA.
With the rapid development of semiconductor devices, Gatti and Rehak first proposed a new type of nuclear radiation detector, i.e., silicon drift detector (SDD), in 1983.[1] The detector has been widely used in some fields such as space exploration, high energy nuclear physics, and nuclear medicine.[2,3] As the resolution accuracy requirements for SDDs increase, how to reduce the noise of devices plays an important role in the manufacturing process. The noise is mainly determined by the leakage current of SDDs, one of the solutions is to optimize the surface passivation to decrease the surface leakage current. This article is focused on how to optimize the surface passivation of an SDD to reduce its leakage current. There are two main ways for surface passivation of semiconductor devices: chemical and field passivation. Both solutions can be achieved by thin film passivation. The common passivation films are mainly SiO2, Al2O3, etc.[4–8] Since SiO2 passivation films have been put forward, they have been widely used to passivate semiconductor devices. However, high temperature (>1000 °C) to fabricate SiO2 films will cause serious degradation of the silicon substrate. Therefore, plasma-enhanced chemical vapor deposition CVD (PECVD), which features a low-temperature process, has attracted a great deal of attention.[9–13] However, SiO2 films deposited by PECVD are not suitable for surface passivation due to their poor interface and bulk properties, although their deposition speed is very fast. On the contrary, Al2O3 thin films exhibit good passivation property for silicon substrates due to their high atomic hydrogen concentrations.[14–16] However, many experiments have shown that the accumulation of H at the interface during the high-temperature annealing process after deposition of Al2O3 films may cause blistering on the surface, which not only destroys the passivation quality but also increases the leakage current of the device.[17–19] Studies have observed that the blistering phenomenon can be suppressed when a thin SiO2 film is grown between Si-Al2O3 since H has a large diffusion coefficient in SiO2 films.[20–22] Hence, a very thin SiO2 film prepared by the nitric acid oxidation of silicon (NAOS) method is adopted. The NAOS SiO2 film has a compact structure, and is proved to be a good material for surface passivation.[23,24] However, its thickness and deposition rate are limited. In order to achieve high insulation property, a certain thickness of the SiO2 film is needed. Therefore, SiO2/Al2O3/SiO2 stacks is designed to satisfy both the requirements of good interface characteristics and the thickness. At present, such a complex dielectric layer has never been studied in SDD fabrication.
Based on the principles of chemical and field passivation, a new passivation structure, SiO2/Al2O3/SiO2 stacks, is proposed in this paper. The first SiO2 film is formed by the NAOS method because of its low interface state and high density of atomic structure. The second SiO2 film is deposited by PECVD considering fast growth rate at low temperature. A thin Al2O3 film deposited by atomic layer deposition (ALD) is introduced into the two SiO2 stacks to improve the surface passivation. Through the capacitance–voltage (C–V) measurements, it is found that the last fixed charge property in the passivation stacks is the positive charge, which acts as field passivation for the n-type substrate. SiO2/Al2O3/SiO2 stacks fully exploits the advantages of chemical passivation. The passivation structure finally achieves a minority carrier lifetime of 5223 μs at an injection level of 5×1015 cm−3.
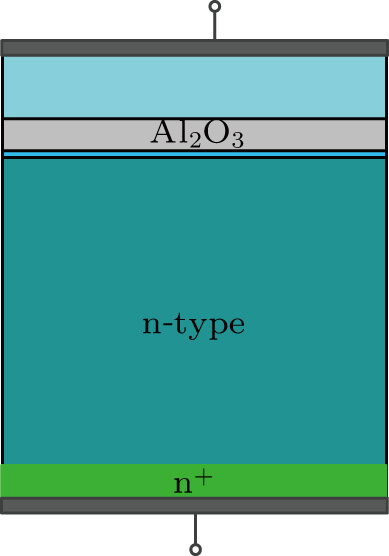
Czochralski-grown 350-μm-thick n-type Si wafers in (100) crystallographic direction, with a resistivity of 3.5 kΩ⋅cm, were used for the passivation experiment. The main process flow is schematically shown in Fig.
In order to further investigate the fixed charge in the passivation stacks, an MIS structure was designed (as shown in Fig.

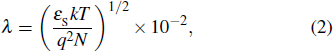
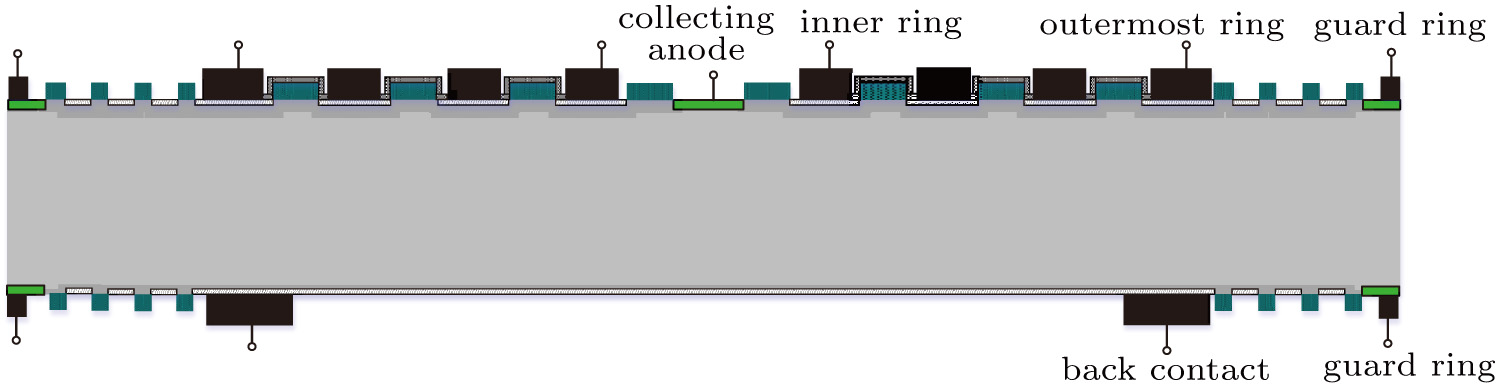
We applied SiO2/Al2O3/SiO2 stacks to the manufacturing of the SDD, the main steps are shown in Fig.
 | Fig. 3. Schematic of the process flow for the fabrication of the voltage dividers, drift rings, guard rings and entrance windows in silicon drift detectors. |
 | Fig. 4. Final structure of the SDD.[26] |
According to the SE results, a thin SiO2 film with a thickness of about 1.2 nm was obtained by the NAOS method. At the beginning, the ALD reaction time was set to 50 cycles, and an about 5.5-nm-thick Al2O3 film was obtained. The thickness of the PECVD-SiO2 film was varied from 300 nm to 600 nm. Figure
Figure
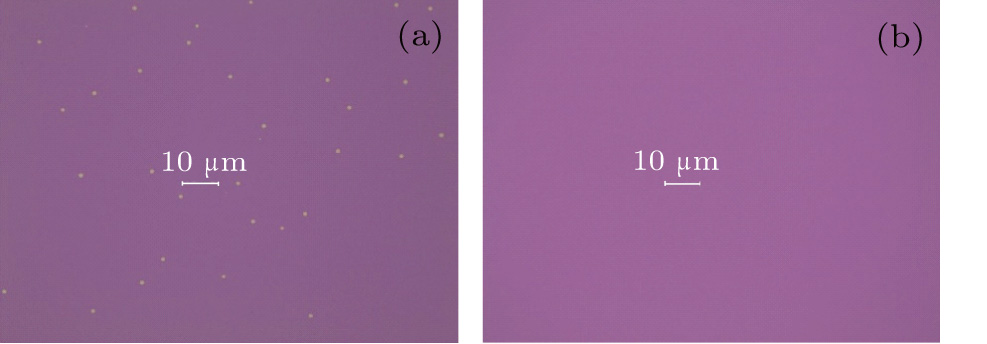 | Fig. 6. Blistering phenomenon of the passivation stacks: (a) Al2O3/SiO2 at 450 °C, (b) SiO2/Al2O3/SiO2 at 450 °C. |
As can be seen from Fig.
Figure
The relationship among the minority carrier lifetime and the first and second annealing temperatures can be seen from Fig.
 | Fig. 9. Relationship between minority carrier lifetime and (a) first annealing temperature, (b) second annealing temperature. |
Figure
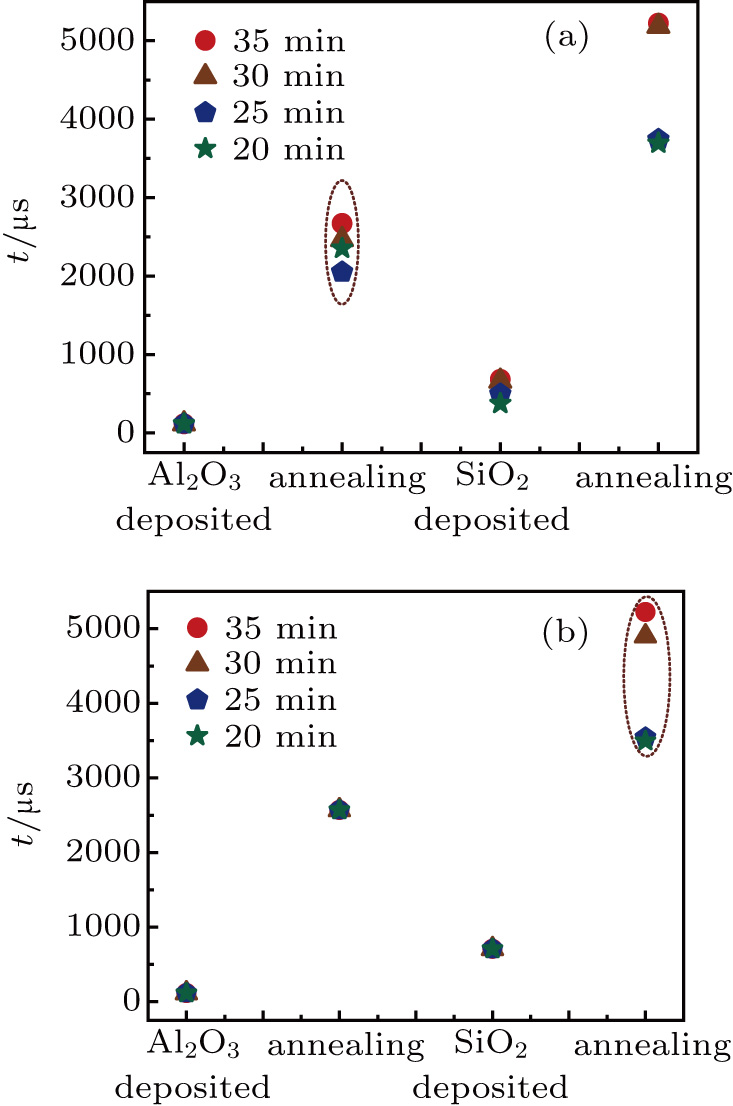 | Fig. 10. Minority carrier lifetime as functions of (a) first annealing time and (b) second annealing time. |
Figures
Figure
Figure
Figure
 | Fig. 15. The relationship between the dark current of the SDD and the outermost ring voltage at different passivation modes. |
However, compared with the SiO2 film grown by the thermal oxygen and PECVD, when SiO2/Al2O3/SiO2 stacks are applied to the SDD, the leakage current is significantly reduced.
| Table 1. Voltage of electrodes. . |
Figure
In order to lower leakage current of SDDs, we have propose a new passivation structure of SiO2/Al2O3/SiO2 stacks. The passivation structure combines chemical and field passivation well. At an injection level of 5 × 1015 cm−3, the minority carrier lifetime reaches 5223 μs. When we apply the new passivation structure to the SDD, leakage current eventually reduces to the nA level, which is greatly contributed to the detection resolution of SDDs.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] |