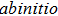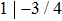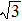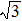Following the discovery of graphene, tremendous interest in other two-dimensional 2D layered materials has been raised.[1–6] These 2D materials have great potentials in applications of functional devices,[7,8] mainly attributed to their tunable properties with various methods, such as through changing the number of layers, chemical components, or by the formation of heterostructures.[1,22] Among the hundreds of 2D materials, transition metal dichalcogenides (TMDs) have drawn much attention recently due to their novel electronic and optoelectronic properties.[7,22] As one member of TMDs, titanium dichalcogenide compounds have been studied extensively, because they show many amazing physical phenomena like charge-density-wave (CDW) state.[22] For example, a real-space periodic lattice distortion in the CDW state of TiSe2 film with interstitial Ti atoms was observed through the scanning tunneling microscope (STM) measurements.[22] Similar phenomenon and possible formation mechanisms about CDW state in the monolayer TiTe2 are also intriguing.[22] Moreover, the spin–orbit coupling or quantum confinement effects may be observed in these 2D TMDs, these novel phenomena are normally hard to observe in their bulk counterparts.[22,22,22,22] Although a large number of TMD materials were fabricated in recent years,[3,22,22,22] new members of 2D TMD materials family with new structures are still desirable, because it holds new possibilities for novel physics and device applications. 2D TiTe2 with 1T configuration has been fabricated and studied recently,[22,22] but 2D TiTe2 with 2H configuration has not been obtained experimentally so far.
Here, we report the fabrication of 2H-TiTe2 monolayer on a Au(111) substrate. The experiments were carried out in an ultrahigh-vacuum (UHV) molecular beam epitaxy (MBE) system with a base pressure of about
 mbar. The Au(111) single crystal surface was cleaned by cycles of argon-ion sputtering and annealing to 760 K until it showed clear diffraction spots in the low-energy electron diffraction (LEED) pattern and atomically clean surface in the STM images. The atomic beams of Te and Ti were evaporated to the as-prepared Au(111) substrate from a Knudsen effusion cell and an electron-beam evaporator, respectively. The process for the fabrication of the TiTe2 film was as follows: Te was deposited at first, while the substrate was maintained at 573 K, then Ti was deposited at the same substrate temperature, and finally the substrate was kept at 573 K for 20 min and then slowly cooled down. By these processes, the TiTe2 film formed on the Au(111) surface with Te as the buffer layer. The as-grown samples were characterized in-situ by LEED to gain the crystal structure and then transferred to the STM chamber to study the surface morphology and detailed geometric properties.
mbar. The Au(111) single crystal surface was cleaned by cycles of argon-ion sputtering and annealing to 760 K until it showed clear diffraction spots in the low-energy electron diffraction (LEED) pattern and atomically clean surface in the STM images. The atomic beams of Te and Ti were evaporated to the as-prepared Au(111) substrate from a Knudsen effusion cell and an electron-beam evaporator, respectively. The process for the fabrication of the TiTe2 film was as follows: Te was deposited at first, while the substrate was maintained at 573 K, then Ti was deposited at the same substrate temperature, and finally the substrate was kept at 573 K for 20 min and then slowly cooled down. By these processes, the TiTe2 film formed on the Au(111) surface with Te as the buffer layer. The as-grown samples were characterized in-situ by LEED to gain the crystal structure and then transferred to the STM chamber to study the surface morphology and detailed geometric properties.
Density functional theory (DFT) calculations were performed using the Vienna
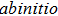 simulation package (VASP). The projector augmented wave (PAW) method was employed and the Perdew–Burke–Ernzernhof (PBE) version of the generalized gradient approximation (GGA) was used. The electronic wave functions were expanded in plane waves with a kinetic energy cutoff of 300 eV. The k-points sampling was 8×3 ×1, generated automatically with the origin at the
simulation package (VASP). The projector augmented wave (PAW) method was employed and the Perdew–Burke–Ernzernhof (PBE) version of the generalized gradient approximation (GGA) was used. The electronic wave functions were expanded in plane waves with a kinetic energy cutoff of 300 eV. The k-points sampling was 8×3 ×1, generated automatically with the origin at the
 point. The structures were relaxed until the energy and residual force on each atom were smaller than 10−5 eV and 0.01 eV/Å, respectively. The STM simulations were performed using the Tersoff–Hamann approach.
point. The structures were relaxed until the energy and residual force on each atom were smaller than 10−5 eV and 0.01 eV/Å, respectively. The STM simulations were performed using the Tersoff–Hamann approach.
Figure 1(a) shows the LEED pattern of the as-prepared sample, and the outer six diffraction spots highlighted by the white circles are originated from the hexagonal lattice of the Au(111) substrate. Apart from these spots, the other diffraction spots can be assigned to the well-ordered film. For more clarity, we sketched a diffraction pattern in reciprocal space as shown in Fig. 1(b), where groups of spots in different sizes and colors are classified in order to describe different structures and domains. Specifically, the diffraction spots are divided to two groups, as shown in Figs. 1(c) and 1(d). Each group of spots is generated by three domains as represented in different colors.
Figure 1(e) and 1(f) show the lattice structures in real space, corresponding to the diffraction spots indicated by the arrows in Figs. 1(c) and 1(d), respectively. The crystalline matrix of the superstructure in Fig. 1(e) is (1,
 , 3). This sketch distinctly shows a commensurate relation between the film and the substrate lattice, which has a superstructure of
, 3). This sketch distinctly shows a commensurate relation between the film and the substrate lattice, which has a superstructure of
 . As discussed in the following, this structure can be attributed to TiTe2 layer on Au(111) substrate with Te as the buffer layer.
. As discussed in the following, this structure can be attributed to TiTe2 layer on Au(111) substrate with Te as the buffer layer.
The diffraction spots shown in Fig. 1(d) are generated by a high-order commensurate structure of (7/4,
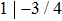 , 1), as shown in Fig. 1(f). They are probably originated from the Te thin film, because excess Te was deposited in the growth process. The lattice period of this structure in real space is ∼0.44 nm, which is consistent with the lattice constant of bulk Te. The crystal unit cell has a little distortion to be commensurate with the substrate lattice.
, 1), as shown in Fig. 1(f). They are probably originated from the Te thin film, because excess Te was deposited in the growth process. The lattice period of this structure in real space is ∼0.44 nm, which is consistent with the lattice constant of bulk Te. The crystal unit cell has a little distortion to be commensurate with the substrate lattice.
To further investigate the surface structure of the sample, STM was used to characterize the surface morphology in real space. Figure 2(a) shows an STM image of the sample surface, in which three domains can be clearly distinguished, as labeled by α, β, and γ. We can also see that the crystal directions are 30°, −30°, and 90°. To characterize the surface structure more clearly, we mapped these three domains separately in detail, as demonstrated in Figs. 2(b)–2(d). It is clear that the superstructure is an oblique structure. The unit cell of the superstructure is marked by a black parallelogram, corresponding to
 superlattice with respect to the Au(111) substrate, consistent with the LEED patterns.
superlattice with respect to the Au(111) substrate, consistent with the LEED patterns.
Figure 2(e)–2(g) are the line profiles along the purple lines in Figs. 2(b)–2(d), in which the period of the superstructure is about 5.0 Å, in agreement with
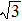 times of the Au substrate lattice constant (
times of the Au substrate lattice constant (
 ). These STM images match with the diffraction spots in the LEED pattern with the
). These STM images match with the diffraction spots in the LEED pattern with the
 structure. The superstructure in Fig. 1(f) deduced from the complicated LEED pattern was not observed in the STM experiments, possibly because STM is a surface-sensitive technique, hard to detect the superstructure underneath the surface layer.
structure. The superstructure in Fig. 1(f) deduced from the complicated LEED pattern was not observed in the STM experiments, possibly because STM is a surface-sensitive technique, hard to detect the superstructure underneath the surface layer.
To understand the STM images, we performed DFT-based calculations and simulations. Figure 3(a) shows a stable structure obtained by the calculations. This is a 2H-TiTe2 monolayer on the Au(111) surface with a buffer Te layer between the TiTe2 film and the substrate, which shows a (
 ) superstructure (red rectangle). The lattice constants of this 2H-TiTe2 are about a=3.69 Å and b=3.28 Å, a little smaller than that of freestanding 1T-TiTe2 of 3.76 Å,[22] which may be induced by the substrate. In order to accommodate the in-plane compression of the lattice constants, the distance between Te atomic layers in the TiTe2 sandwich structure is elongated in the z direction to be 4.13 Å, while this distance in freestanding 1T-TiTe2 is only 3.47 Å.[22] Figure 3(b) is the simulated STM image of this relaxed structure. Besides (
) superstructure (red rectangle). The lattice constants of this 2H-TiTe2 are about a=3.69 Å and b=3.28 Å, a little smaller than that of freestanding 1T-TiTe2 of 3.76 Å,[22] which may be induced by the substrate. In order to accommodate the in-plane compression of the lattice constants, the distance between Te atomic layers in the TiTe2 sandwich structure is elongated in the z direction to be 4.13 Å, while this distance in freestanding 1T-TiTe2 is only 3.47 Å.[22] Figure 3(b) is the simulated STM image of this relaxed structure. Besides (
 ) superstructure (red rectangle),
) superstructure (red rectangle),
 structure (black parallelogram) is also displayed, because the electronic states of the top Te atoms merge. Figure 3(c) is an experimentally obtained high resolution STM image, which shows a good agreement with the simulated result in Fig. 3(b), indicating the validity of the structural model with 2H-TiTe2 monolayer on Au(111) and Te as buffer layer. We also calculated the structure with 1T-TiTe2 as the top layer and simulated the STM image. However, the results are inconsistent with the (
structure (black parallelogram) is also displayed, because the electronic states of the top Te atoms merge. Figure 3(c) is an experimentally obtained high resolution STM image, which shows a good agreement with the simulated result in Fig. 3(b), indicating the validity of the structural model with 2H-TiTe2 monolayer on Au(111) and Te as buffer layer. We also calculated the structure with 1T-TiTe2 as the top layer and simulated the STM image. However, the results are inconsistent with the (
 ) superstructure observed in the LEED and STM experiments.
) superstructure observed in the LEED and STM experiments.
In summary, we have successfully synthesized 2D TiTe2 layer with 2H configuration, which is a new member of the TMDs family, on a Au(111) surface by MBE method. STM and LEED characterizations combined with DFT calculations demonstrate the formation of
 superlattice of the TiTe2 monolayer with Te as buffer layer on the Au(111) substrate. This work may help to enrich the understanding of the superstructures of 2D layered materials and provides a new candidate in 2D materials family to explore novel physical properties and related applications.
superlattice of the TiTe2 monolayer with Te as buffer layer on the Au(111) substrate. This work may help to enrich the understanding of the superstructures of 2D layered materials and provides a new candidate in 2D materials family to explore novel physical properties and related applications.