† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51532006), the Fund from Shanghai Municipal Science and Technology Commission (Grant No. 16DZ2260600), the 111 Project of the Ministry of Education, and the Fund from the National Bureau of Foreign Experts (Project No. D16002).
Grain-boundary (GB) structures are commonly imaged as discrete atomic columns, yet the chemical modifications are gradual and extend into the adjacent lattices, notably the space charge, hence the two-dimensional defects may also be treated as continuum changes to extended interfacial structure. This review presents a spatially-resolved analysis by electron energy-loss spectroscopy of the GB chemical structures in a series of SrTiO3 bicrystals and a ceramic, using analytical electron microscopy of the pre-Cs-correction era. It has identified and separated a transient layer at the model Σ5 grain-boundaries (GBs) with characteristic chemical bonding, extending the continuum interfacial approach to redefine the GB chemical structure. This GB layer has evolved under segregation of iron dopant, starting from subtle changes in local bonds until a clear transition into a distinctive GB chemistry with substantially increased titanium concentration confined within the GB layer in 3-unit cells, heavily strained, and with less strontium. Similar segregated GB layer turns into a titania-based amorphous film in SrTiO3 ceramic, hence reaching a more stable chemical structure in equilibrium with the intergranular Ti2O3 glass also. Space charge was not found by acceptor doping in both the strained Σ5 and amorphous GBs in SrTiO3 owing to the native transient nature of the GB layer that facilitates the transitions induced by Fe segregation into novel chemical structures subject to local and global equilibria. These GB transitions may add a new dimension into the structure–property relationship of the electronic materials.
Grain-boundaries (GBs) in SrTiO3 often serve as a model system for interface studies owing to its cubic structure at ambient conditions that minimizes the anisotropic effect on interfacial planes, while the mixed cations enable more opportunities and wide ranges to control or tune the electric and dielectric properties at the interface. High-resolution transmission electron microscopy (TEM) analysis, which traces as well as reduces the quasi-two-dimensional imperfection down to its structural origin,[1–3] treats interfacial structures as discrete atomic columns. In this era of aberration-corrected TEM using a scanning probe (STEM), the analysis of interfacial structures reaches unprecedented lateral resolution, not only in atomic arrangements but also in chemical identity down to each column.[4] However, the GBs as two-dimensional (2D) imperfections are still the singular defects under the thermodynamic landscape, which induce chemical variations especially by segregation of impurities or dopants, owing to their lower chemical potentials than the matrix. In other words, there is a chemical structure of a finite scale around such internal interfaces that is distinctive to the interrupted lattice above the atomistic scale.
Indeed, impurity segregation was found to turn the GB structures into amorphous layers with equilibrium width of ∼ 1 nm in certain ceramic compounds.[5,6] A GB “complexions” scheme was later proposed to treat a wide range of GB structures as interfacial “phases” to expand the segregation picture from sublayer to multilayer adsorption, thus rationalizing the amorphous GB as originated from interfacial transition to drive the accelerated grain growth.[7,8] In parallel, a spatially-resolved electron energy-loss spectroscopy (EELS) analysis had identified a specific oxynitride structure for amorphous GB layers in terms of bonding, composition, and width in nitride ceramics.[9,10] Such chemically stable amorphous structure renders the extended GB layer with predictable electro-chemical affinity to serve as native host to segregated cations. In comparison, the amorphous GBs as silica-based layers in carbide and oxide ceramics largely exhibited a transient nature, which could either promote adjacent minor phase or induce anisotropic grain growth.[11–13]
For SrTiO3 as the ternary compound, chemical structures of the model quasi-2D GB and general GB have both been studied extensively, especially the effect and distribution of segregated accepter or doner but under rather different approaches and resolutions. The model GB was focused mainly into core-structure at subatomic level, while the latter is more into the segregation behavior at a nanometer scale. They both merge to the key issue of charge transfer or re-distribution; one performs in a bottom-up approach to build up the electronic map while the other starts top-down to follow the thermo-chemical origin. The concept of space charge has emerged as an outcome of redistribution of defects to account for charge balance at and near such interfaces, which creates inherent non-stoichiometry either as source or sink to accommodate the excessive defects as demonstrated in Fig.
 | Fig. 1. (color online) Schematic diagram of space charge model for GB non-stoichiometry to accommodate intrinsic defects in SrTiO3; adapted from Refs. [14] and [15]. This extended configuration of GB chemistry receives acceptor dopant by their segregation to the space charge zones. |
In 1990s, analytical electron microscopy (AEM) had developed into a matured methodology suitable for probing extended chemical structure of the GB, especially by spatially-resolved EELS. This spectroscopy is sensitive to both the elemental and electronic distribution at a subnanometer scale, especially when such quantitative inquisition could reveal inherent correlation between the chemistry and spatial scale, although it remains largely a continuum approach.[19] Nevertheless, it would be handy to associate with the atomistic studies via quantification of a collection of chemical parameters for the probed interface,[20] hence is still useful in this age of aberration-corrected STEM. In this review of AEM analysis, an extended abstract for a conference[21] and an unpublished internal report from the pre-Cs-correction age have been combined. I present a comparative study of chemical structures of model Σ5 GBs and a general GB with amorphous film in SrTiO3, from intrinsic interfacial layer to associated transition induced by acceptor segregation, ready for further verification by dielectric properties of individual GBs or calculated using integrated modeling.
Dopant and defect segregation to GB is an inherent chemical property of polycrystalline materials associated with intrinsic and extrinsic chemical defects. This constitutes a thermodynamic variable that could be measured as excess of a chemical constituent segregated to GB above its concentration in the lattice, even by an inquisition probe in the order of 10 nm. The “spatial-difference” technique was developed initially to reveal the weak segregation that often buried under huge background, especially in EELS analysis. This technique improved the detection limit below one atom per nm2 area of an interface or GB to enable direct correlation of the excess with local atomic structure and chemical bonding.[22] To probe into the native amorphous layer at ceramic GBs, this technique was further developed into a quantitative methodology for GB chemistry, starting by identifying characteristic EELS near-edge structure (ELNES) signals specific to GB layers.[19] By systematic spectral processing via “ELNES separation”, full spectrum exclusively for a GB layer could also emerge; hence, its chemical composition and associated width were concurrently obtained, even when the effective probe size was significantly larger than GB layer.[10] However, such an analysis leads to loss of detailed information inside the GB layer, hence it is ideally applicable for the GB layer with a distinctive and uniform structure such as an amorphous film. On the other hand, such interactively identified GB layer may or may not be one useful for the “complexions” scheme that focuses onto structural changes induced by layered transition of chemical adsorbates segregated to GB core.[8] Hence, it minimizes or even trivializes the inherent properties from the adjacent lattices especially the effect from their misorientation relationship, which may be true only in the case of amorphous GB layer.
“ELNES separation” methodology applied to the GBs in SrTiO3 is effectively a cut-off method for continuum tails to identify a GB layer with significant structural modification manifested in characteristic bonding including, but not limited to, the GB core. Such distinctive bonding might change gradually within this GB layer of finite width, no matter whether it is associated with the segregated dopant that might reconstruct the core structure, or with the intrinsic defects that may not alter the structural coordination. Extrinsic defects induced by dopant segregation could combine both into a further extended GB layer, especially for acceptor that is expected to segregate to the space charge zone that expands to tens of nanometers outside the GB layer (Fig.
Therefore, a series of GBs in SrTiO3 were selected for this spatially-resolved EELS analysis for the comparative study of the GB chemical structures, namely Σ5 GBs from bicrystals and amorphous GBs in a ceramic material. These bicrystals were created by cutting along the (310) plane of single crystals and reorienting the two pieces into 36.5° symmetric boundaries around the common [001] direction. The bicystals were then reheated under hot-pressing, and were doped with 0 wt %, 0.1 wt %, and 0.5 wt% of Fe2O3 respectively before purchasing from commercial source (Wako Busan, Tokyo, Japan); the ceramic SrTiO3 was hot-pressed and doped with 0.8 mol% of Fe2O3; more details about materials processing can be found in an earlier publication.[16] The TEM specimens were mechanically sliced and ion-milled until perforation. The experiments were performed in a dedicated STEM of Vacuum Generator (model HB501, Cambridge, UK) at an accelerating voltage of 100 kV, which offered an analytical probe with sufficient beam current of diameter less than 1 nm. A parallel electron spectrometer (model 666, Gatan Co., Pleasanton, California) was used to acquire EELS spectra. This STEM/EELS system provided an energy resolution of 0.7 eV for ELNES investigation at energy dispersions of 0.3 eV/channel–0.5 eV/channel at the photodiode array, while it degraded to 1 eV for compositional measurements at the dispersions of 1.0 eV/channel.[19] When acquiring an EELS spectrum, a low-angle annular dark-field (LAADF) detector was placed to limit EELS collection within an angle of 6.5 mrad. In the meanwhile, this detector could acquire a LAADF image in an angular range of 6.5 mrad–20 mrad along with a high-angle annular dark-field (HAADF) detector image in the high-angle range of 35 mrad–160 mrad, during the “spectrum-image” acquisition mode of EELS spectra by the scanning probe.[19]
The methodology of spatially-resolved EELS analysis of GB includes two different modes in experimental setups, followed by systematic spectral processing, as demonstrated with an SrTiO3 bicrystal to reveal the chemical structure of a Σ5 symmetric GB viewed along the common [001] zone-axis [Fig.
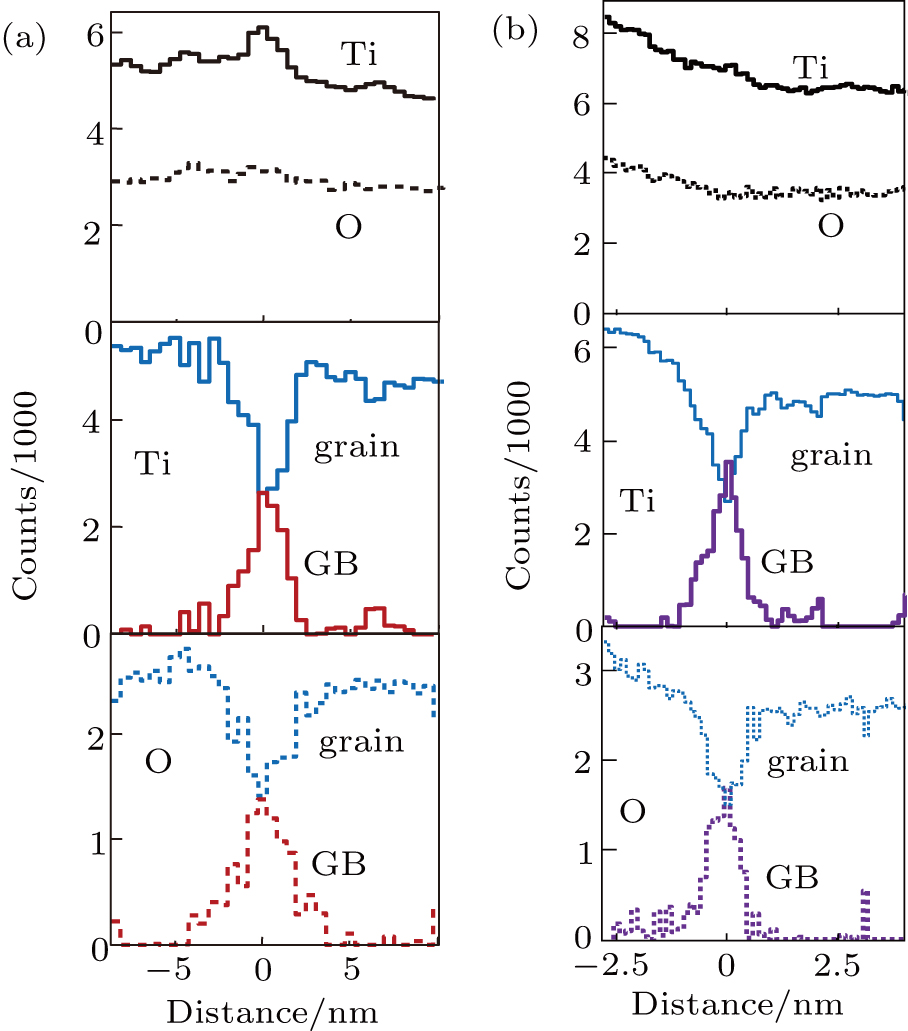
To gain a dedicated spectrum exclusively for the GB layer, off-line spectral processing is necessary for both modes in a similar way as demonstrated for the two-frame mode in Fig.
This processed ELNES patterns of Ti–L2,3 and O–K edges, corresponding to a finite layer of intrinsic Σ5 GB, are shown in Figs.
This spatially-resolved EELS methodology can derive further chemical information from this GB layer, which is better presented by the off-line processing for spectrum-profiling as demonstrated in Fig.
To derive more information from this GB layer, such severe probe broadening should be modeled for probe shape before deconvolution. Alternatively, this could be removed by a simple deconvolution method that requires no modeling of probe intensity profile,[10] as demonstrated in Fig.
| Table 1. Chemical parameters of GB layers obtained using the spatially-resolved EELS analysis. . |
Segregation of Fe to the GB was successfully detected only for high levels of Fe2O3 doping, either at 0.5 wt% for bicrystals or 0.8 mol% for ceramics, measured as excess as listed in Table
As shown in Figs.
Indeed, O–K ELNES patterns from these Σ5 GB layers exhibit trends of chemical change owing to increase in percentage of Fe2O3 dopant, as shown in Fig.
On the other hand, another chemical structure, which is even more similar to rutile structure as revealed in Fig.
These two chemical structures for Fe-segregated Σ5 GB might be further correlated with each other in a further transition. The heavily distorted bonding and clearly higher density of Ti–O6 coordination stores tremendous amount of strain into this GB layer under hot-pressing, as witnessed by 40% and 20% increase for Ti and O, respectively, within a width of 1.1 nm. The first chemical structure of strained GB has a mixture of Ti–O6 linkages in both the corner-sharing and edge-sharing configurations, ready to release the strain from the confined layer especially during the thinning process for preparing TEM specimens. The second chemical structure of this “bad” GB is probably a result of such a release of GB strain, not only due to lack of concentration excesses, but also because of much thinner GB layer owing to the possible de-mixing of corner-sharing and edge-sharing Ti–O6 configurations. This leaves the latter to fill one third of the spacing between two unaltered grains. Indeed, along this GB and hundreds of nanometers far from the area for the “bad” GB structure, visible cracks were observed. In other words, the strained GB structure may be half a transition to create mixed Ti–O6 configurations into the strained, extended GB layer confined into no more than 3 unit-cells.
In light of the revelation of chemical transition for Σ5 GB induced by Fe segregation, chemical structure of the general GB in SrTiO3 ceramic was also analyzed using this spatially-resolved EELS analysis. GB structures in ceramics are usually in unknown misorientations between two grains, which form either in an amorphous film of ∼ 1 nm thickness, or without any amorphous or crystalline film. One such GB film is observed in both LAADF and HAADF images as shown in Fig.
EELS profiling across this amorphous film reveals more characteristics of its chemical structure, as demonstrated in Fig.
The detection of Fe dopant segregated within a layer of the GB, at both coherent Σ5 type and general amorphous film, signifies that the space charge layer initiated by the acceptor segregation does not exist in SrTiO3, hence there is no oxygen deficiency in expected configuration at GB core to be balanced by the space charge. Instead, the GB core structure expands by modification of Ti–O6 linkage network to alter the native chemical structure of 2–3 unit cells into extended GB layer, which concurrently aids taking in more Fe dopant while leaving out some Sr ions due to reduced spaces between the edge-sharing Ti–O6 coordination mixed with corner-sharing configurations. This situation was compared with full spectra of different chemical structures including Ti–L2,3, O–K, and Fe–L2,3 edges, as presented in Fig.
In the meanwhile, Fe segregation witnesses the transitions by following their randomness in Ti–O6x network as measured in Fe:Ti ratio, which increases more than twice from the mixed linkage in the constrained Σ5 GB to the mixture of octahedral and tetrahedral coordination in the confined amorphous film, and a further four times in the unconfined glass pocket. This behavior for Fe dopant indicates a mode change from segregation to solution in the random network of Ti–O6/Ti–O4. This is indeed the favorable chemical configuration to accommodate iron dopant since Fe3+ ions act as network-builders and modifiers to replace both Ti4+/Ti3+ and much bigger Sr2+ ions, especially for the latter in the film, as such randomness tends to decrease the number of coordinated oxygen ions by cation. The chemical composition of the glass pocket is TiFe0.55O2.1 without including Sr, or Ti1.43Fe0.79O3 based on Ti2O3, whereby Ti3+ and Fe2+ ions dominate this chemical structure to maintain the stoichiometry with only one-sixth of Fe3+ ions among all iron solutes. The chemical formula of amorphous film is estimated as Ti0.83Sr0.25Fe0.1O2 based on TiO2, and it turns to Ti1.25Sr0.95Fe0.18O3 based on Ti2O3 with only Fe2+. In other words, Sr:Ti ratio jumps from 0.3 to 0.86, or nearly three times, by reducing both the solvent and solute cations. The ratio should be in between the two values; 20% lower concentration of Ti cation may give a further estimation, which must be obtained by modeling or experimental references. In addition, Sr deficiency in the GB film is measurable as negative excess to GB by EELS or EDS analysis, which may fully address the chemical composition of GB film.[9,24] In Fe-segregated Σ5 GB layer found also with excessive titanium and oxygen, the chemical composition is TiSr0.65Fe0.06O2.7 with Ti4+ and Fe3+ ions, or TiSr0.68Fe0.06O2.7 with Fe2+ ions. It is impossible to match Sr level with that of Ti or close since this would raise Ti3+ ions to 70%. The opposite case of Ti4+ dominance should be true because the heavily strained, more compact Ti–O6 network can take much smaller Fe ions to fill some Sr sites, while leaving some other Sr sites vacant to resettle for stoichiometry.
Therefore, Fe segregation modifies the GB structure and expands the core into a layer of 3 unit cells with distinctive chemical structure, effectively inducing a GB transition as schematically depicted in Fig.
Such two transitions (or half-transitions) between these three GBs of SrTiO3 are conceptual and may not realize on the same GB in experiments. This is because there is a scale gap between the local and global equilibria, one reached only a few unit cells out of the GB plane while the other established between correlated thermodynamic identities across the grain scale. The space charge picture (Fig.
On the other hand, the scheme of the GB complexions was also established to bridge the global and local equilibria by taking the GBs directly as 2D phases to merge into bulk phase diagrams. This may go too far in the opposite direction of the space charge picture, especially for complexion transition to initiate abnormal grain growth hence to dictate development of ceramic microstructures.[8] The GB transition in ceramics must involve TP to reach global equilibrium by connecting the two scales across and along the GB, no matter how the grain was grown in SrTiO3 ceramics as well as in structural ceramics.[9,11,13,24] Facet transition and nanoprecipitation could also occur at the same GB planes, both before and after the grain growth.[27–29] However, such a variety of locally or globally equilibrated chemical structures at the GBs of SrTiO3 can help us to better understand the dielectric properties associated inherently with them, often imprinted into the formation of these chemical structures when interfacial charge could as well play a role.[30]
This spatially-resolved analysis from the pre-Cs-correction age probes into the electrochemical structure of representative SrTiO3 GBs especially those connected to their immediate neighborhood, although in the absence of precise information down to atomic columns at the GB core. In a continuum approach, this study counters the picture of the space charge induced by the accepter dopant to compensate for non-stoichiometry at the GB core. Instead, distinctive chemical structures, ∼3-unit cells wide, emerge for both coherent Σ5 GB and amorphous GB film. These are effectively created because of two transitions of chemical structures induced by Fe segregation, or two half-transitions, distinguished only by local or global chemical equilibria re-established within this GB layer. Such a quantitative study of chemical distribution to associate it with width makes the resultant chemical information compatible to further detailed studies of electronic structures of the GB structures, especially with theoretical modeling to reveal the chemical interaction at the subatomic scale.
Although this review concentrates only on two cases representing model and general GBs, combining structural and chemical information across various scales should be followed and widened in future TEM studies of the GB structures in SrTiO3 as well as other materials. Even for this work using a low-precision STEM of pre-Cs-correction age, EDS analysis of Sr-deficiency might well complete such analysis of interfacial chemical structures. Theoretical modeling for ELNES patterns will provide further information about structures and physical phenomena within these GB layers, which could be associated with an independent structural analysis under higher resolution than the chemical analysis. In the present age of Cs-corrected TEM, this extended GB picture to include the space charge effect could be further extended to spin ordering effect induced by the interfacial chemical structure, especially in combination with the electron magnetic circular dichroism technique that approaches the nanometer scale.[31] Inclusion of the interfacial magnetic structure may lead to a “hierarchical” interfacial structure with different scales for distributions of segregation, vacancy, charge, and spin order, hence reaching full analysis of interfacial structures. Furthermore, such a variety in chemical structures of the GBs signifies that it is necessary to tune and control dielectric properties by more sophisticated modeling to rationalize systematic experiments in electronic materials, especially in cases across atomic and nanometer scales.[4,18] This comprehensive study of analytical TEM opens a new possibility to establish collective correlation with the electrical behavior of an assembly of GBs via in situ and/or exsitu experiments, hence expanding the structure-property relationship into a multiscale landscape.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] |