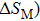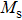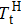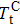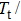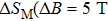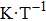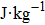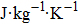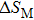† Corresponding author. E-mail:
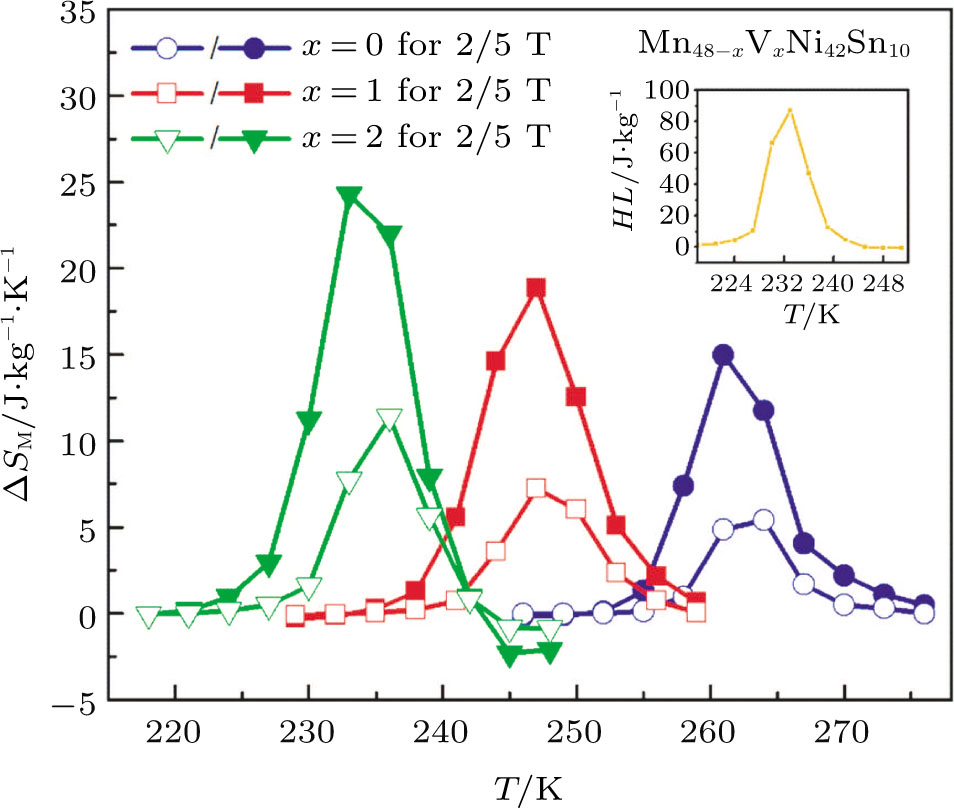
In this work, we tuned the magnetostructural transformation and the coupled magnetocaloric properties of Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) ferromagnetic shape memory alloys prepared by means of partial replacement of Mn by V. It is observed that the martensitic transformation temperatures decrease with the increase of V content. The shift of the transition temperatures to lower temperatures driven by the applied field, the metamagnetic behavior, and the thermal hysteresis indicates the first-order nature for the magnetostructural transformation. The entropy changes with a magnetic field variation of 0–5 T are 15.2, 18.8, and 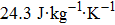
Magnetic refrigeration has drawn great attention from researchers because of its advantages over the conventional cooling technique, such as the compact size, low mechanical vibration, environmental friendliness, and high efficiency.[1–3] Materials exhibiting the large magnetic entropy changes (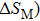
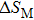
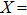
The magnetocaloric effect in NiMn-based magnetic shape memory alloys is generated from the magnetic-field-induced first-order magnetostructural transformation from martensite to austenite.[21–23] According to Liuʼs report, the adiabatic temperature change with a magnetic field variation of 0–2 T is as large as −6.2 K in Ni–Mn–In–(Co) alloy,[24] which is larger than the magnetocaloric effect of Gd. Additionally, due to the fact that the magnetostructural transformation temperature is sensitive to the valence electron concentration (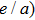
In this work, we investigate the magnetostructural transformation and magnetocaloric properties of Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) ferromagnetic shape memory alloys. The martensitic transformation temperature decreases when Mn is substituted by V. High field M–T and isothermal magnetization curves confirm the magnetic-field-induced magnetostructural transformation. The tunable martensitic transformation temperature, enhanced magnetic field driving capacity, and large magnetic entropy changes are observed.
The polycrystalline Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) alloys were prepared by arc melting the appropriate amount of constituents (99.98% purity) in an argon atmosphere using a water-cooled copper crucible. The ingots were remelted for three times to ensure the homogeneity. After that, the ingots were annealed at 1073 K for three days in vacuum quartz tubes, and then quenched into cold water. X-ray energy-dispersive spectroscopy (EDS, Thermo system 7) was used to determine the elemental composition of the samples. The structural transitions were investigated by differential scanning calorimetry (DSC, Mettler Toledo) with a rate of 10 K/min during heating and cooling cycles. The crystal structures of samples were identified by x-ray diffraction (XRD, Bruker, D8 Advance) using Cu-Kα radiations. Magnetic measurements were carried out on physical property measurement system (PPMS, Quantum Design, Dynacool) and magnetic property measurement system (MPMS-7, Quantum Design). To avoid the irreversibility, the isothermal magnetization curves (M–B) were measured using a so-called loop method.[36]
Figure
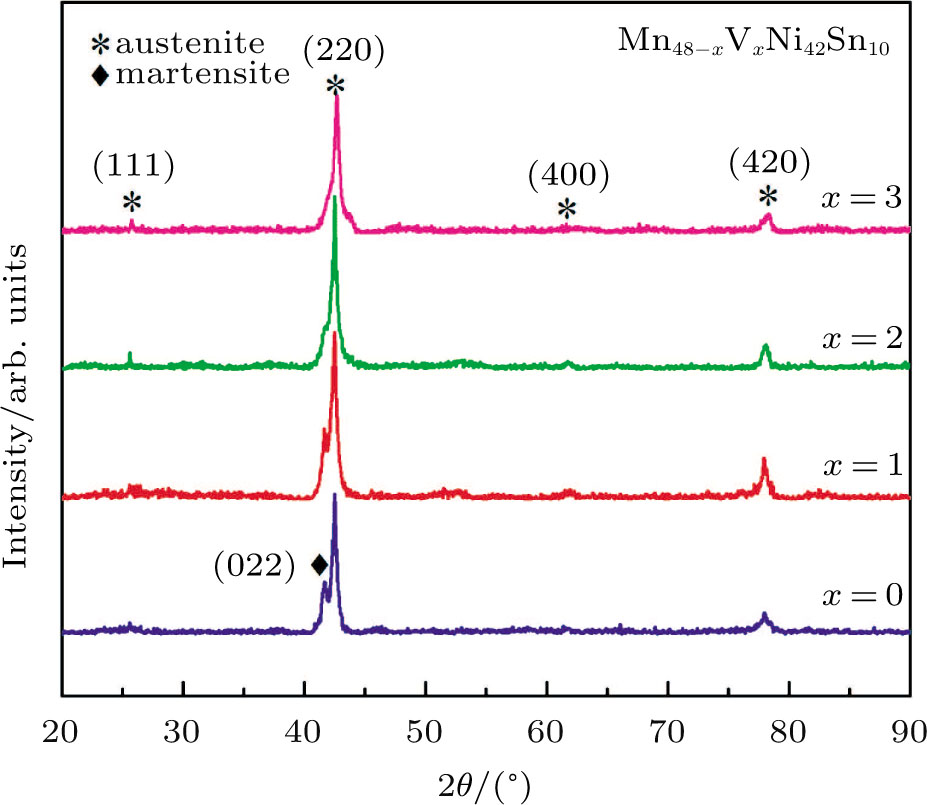 | Fig. 1. (color online) XRD patterns of Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) alloys at room temperature. |
Figure 
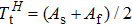
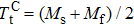
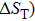
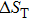
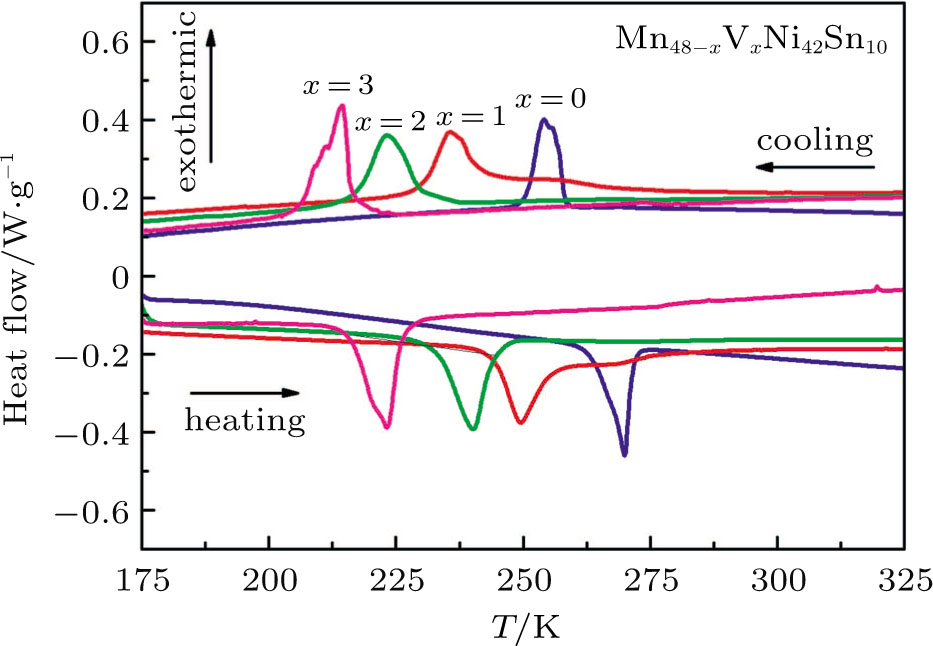 | Fig. 2. (color online) DSC curves of Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) alloys during heating and cooling processes. |
| Table 1.
The measured elemental compositions and corresponding electronic concentrations for the present Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) samples. . |
The temperature dependence of magnetization (M–T) curves for Mn48−xVxNi42Sn10 (x = 0, 1, and 2) alloys were measured to investigate the magnetic phase transitions. Figure 
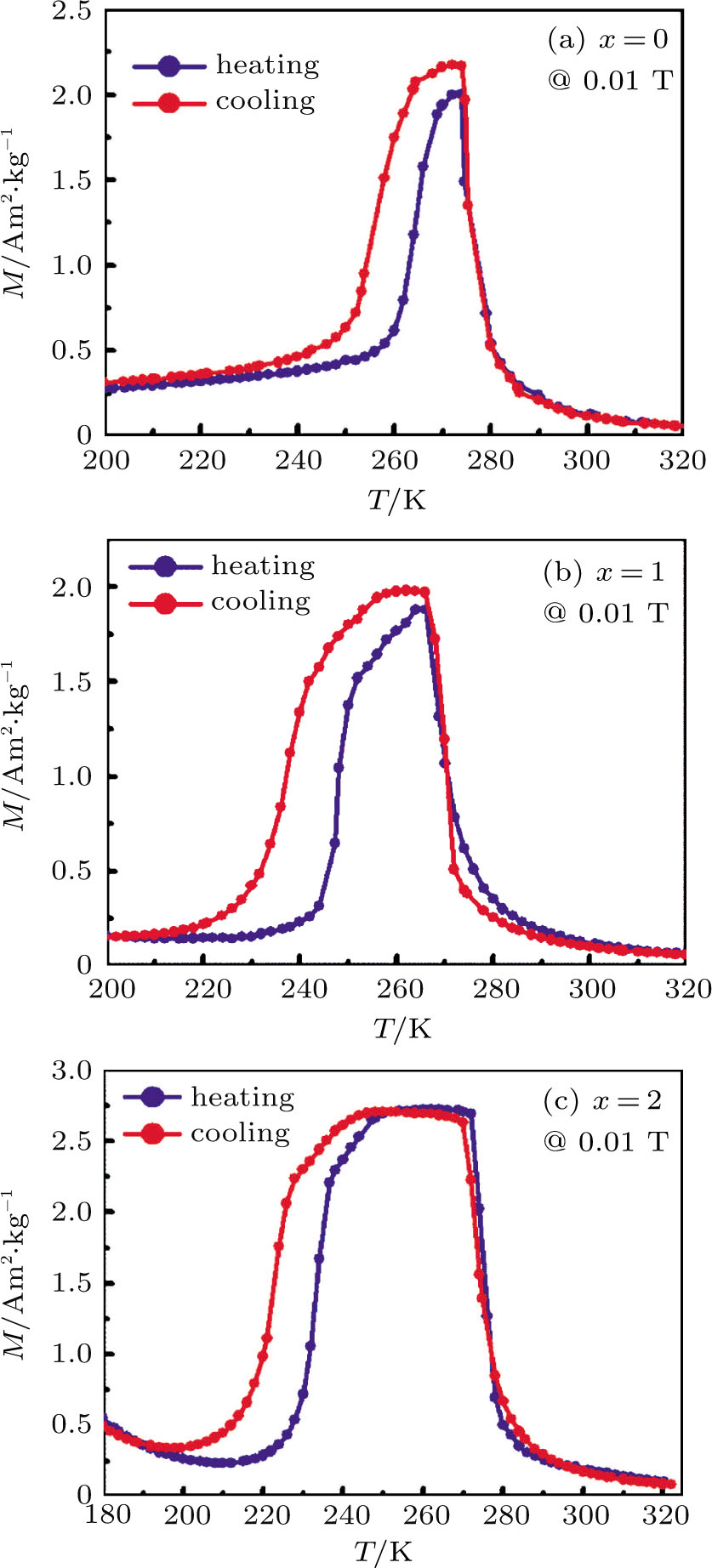 | Fig. 3. (color online) The temperature dependence of magnetization (M–T) curves for Mn48−xVxNi42Sn10 (x = 0, 1, and 2) alloys under an applied field of 0.01 T. (a) x=0, (b) x = 1, and (c) x = 2. |
| Table 2.
Phase transformation temperatures, effective refrigeration capacity (RCeff), field driving capacity (d |
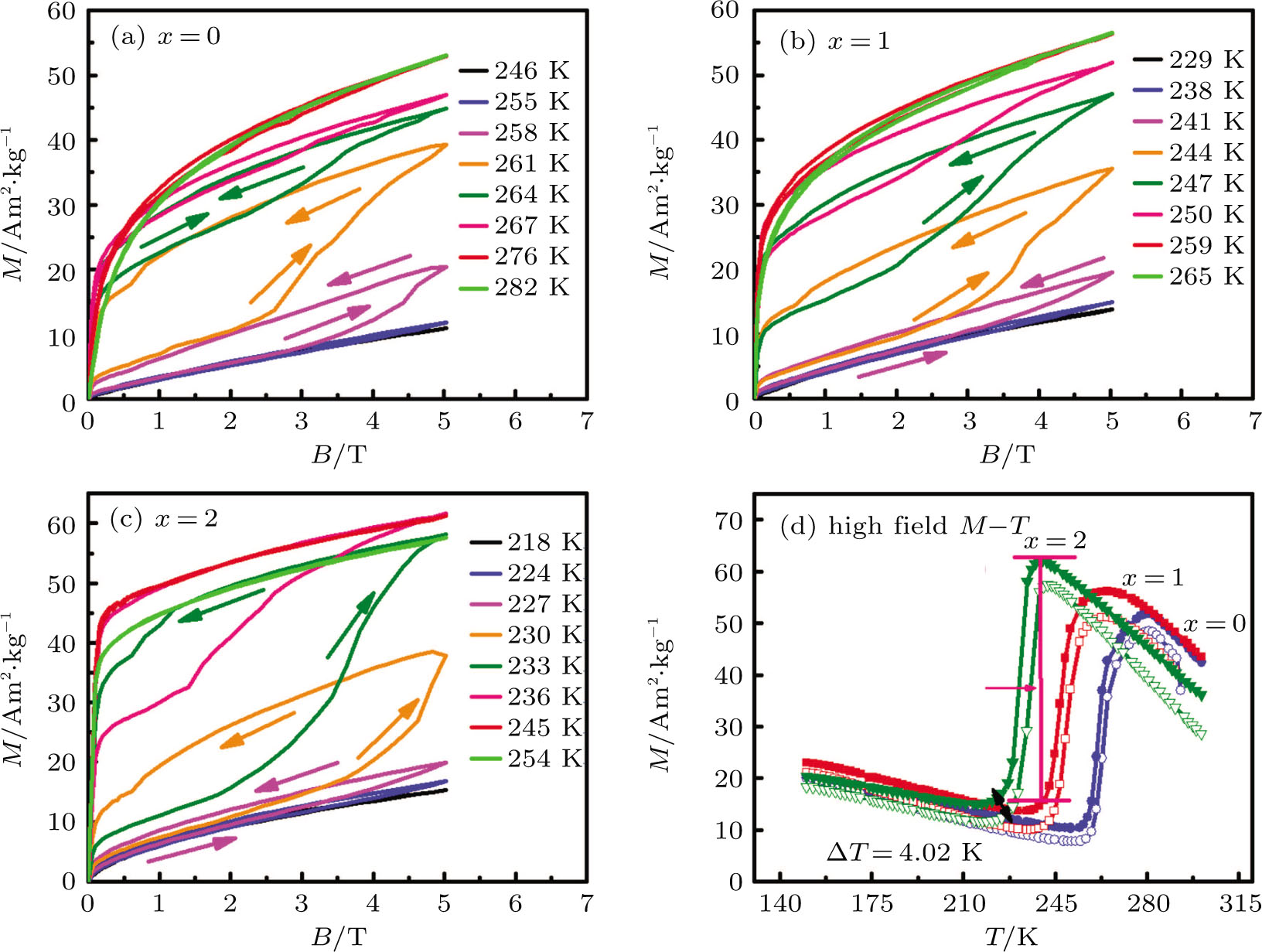
In order to investigate the magnetic-field-induced structural transformation, the isothermal magnetization (M–B) curves for Mn48−xVxNi42Sn10 (x = 0, 1, and 2) alloys in the field of 0–5 T were measured by the so-called loop method.[36] Initially the samples were cooled down to complete the martensitic state and then slowly heated to the target temperature before starting each M–B measurement. As shown in Figs. 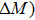

Owing to the magnetization difference between the martensite and austenite, the structural transformation can be driven by the magnetic field. With increasing the magnetic field from 3 to 5 T, the M–T curves of Mn48−xVxNi42Sn10 (x = 0, 1, and 2) alloys shift to the lower temperature, as shown in Fig. 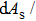
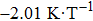
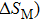
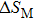
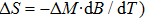
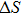
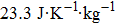
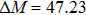
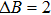
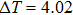
According to the M–B curves, the 
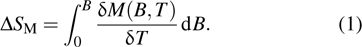 |
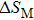
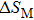
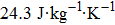
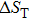
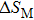
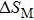
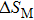
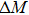
The effective refrigeration capacity (RCeff) of Mn48−xVxNi42Sn10 (x= 0, 1, and 2) alloys is calculated by subtracting the average hysteresis loss (HL) from the refrigeration capacity (RC) values. Here, the value of RC is obtained by integrating the area under 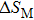
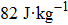
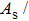
The magnetostructural transformation and the coupled magnetocaloric properties for Mn48−xVxNi42Sn10 (x = 0, 1, 2, and 3) ferromagnetic shape memory alloys are investigated using both structural and magnetization measurements. By replacing Mn with V, the martensitic transformation temperature can be tuned within a wide temperature range. The magnetic-field-induced magnetostructural transformation is confirmed by the high field M–T curves and isothermal magnetization curves. The observed peak values of 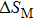
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] |