† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 21374074, 21422404, and U1532108) and the Undergraduate Training Program for Innovation and Entrepreneurship of Soochow University, China (Grant No. 2016xj010).
Imaging-guided cancer therapy provides a simultaneous tumor imaging and treatment, which helps to eliminate the excessive toxicity to the healthy tissues. For this purpose, multifunctional probes capable of both imaging and curing are needed. In this work, we synthesize water-soluble silicon quantum dots (Si QDs) smaller than 5 nm. Such Si QDs are used for delivering the hydrophobic drug phthalocyanine (Pc). The as-prepared Si/Pc nanocomposite particles show efficient transmembrane delivery into cells and feasible biocompatibility. Moreover, these composite particles emit dual-channel fluorescence signals even after cellular internalization and demonstrate robust photostability in the Si channel. More interestingly, the Si/Pc composite particles show efficient photodynamic therapy effects against tumors both in vitro and in vivo.
As an advanced therapeutic formula, imaging-guided cancer therapy (IGCT) provides targeted treatment of tumors under the guiding of the labelling probes, in order to eliminate the excessive toxicity to the surrounding normal tissues and to promote the therapeutic efficiency against cancer cells.[1–3] Particularly, the fluorescence imaging-guided cancer therapy (FIGCT) has attracted great interest due to the wide and growing applications of fluorescence in fundamental life sciences, biomedicine and bioengineering fields.[4–6] Multifunctional probes are widely needed for both imaging and therapeutic capabilities.
A variety of strategies have been developed for multifunctional probes, such as the FITC-labeled SiO2 nanospheres, fluorescent protein-doped gold nanoparticle capsules and fluorescent quantum dot-loaded phospholipid micelles.[7–9] However, these materials are facing challenges including the complicated procedures for carrier preparation and drug encapsulation, the large size (mostly 25–100 nm), limited cell uptake efficiency, and/or poor photostability. The semiconductor quantum dots, such as CdSe and CdTe nanoparticles, demonstrate robust fluorescence and improved photostability compared with most commercial fluorescence probes.[10–15] Nevertheless, they show inevitable cytotoxicity. Other types of particles, such as Au and Ag nanoparticles, were also developed for photothermal therapy of tumors or antimicrobial purposes.[16,17] On the other hand, compared with these heavy metal-based particles, the silicon nanostructures, including nanodots, nanowires and nanospheres, have shown feasible biocompatibility.[1,18–22] Moreover, the surface of the Si nanostructures can be easily functionalized for special purpose such as targeted tumor therapy.[18,23–25] Thus, they have been developed for biosensing, bioimaging, and tumor therapy applications. Water-dispersible Si quantum dots (Si QDs) with relatively high quantum field (~ 25%) have recently been synthesized by the reduction of (3-Aminopropyl)trimethoxysilane in aqueous solution.[12] The as-obtained Si QDs have a small size below 5 nm, which ensures them to be renally cleared in vivo.
Here, we would like to testify the applications of such Si QDs for FIGCT purpose. Phthalocyanine (Pc) is selected as a representative drug which has shown photodynamic therapy (PDT) effect of tumors.[26–29] As most of the anti-tumor drugs, Pc is water-insoluble and needs to be delivered into tumor cells with water-dispersible carriers. Under the irradiation of light at a certain wavelength, Pc is able to emit reactive oxygen species (ROS) to kill the tumors. Furthermore, the fluorescence emission of Pc in the biological window range with a near-infrared (NIR) wavelength promises its applications in bioimaging.
In this paper, we will show that the hydrophobic Pc can be stably loaded to the Si QDs via facile preparation such as physical adsorption or chemical binding. The as-obtained Si QD-based composite nanoparticles show robust dual-channel fluorescence signals for imaging and obvious PDT effect of tumors both in vitro and in vivo. This work promises the possibility of the Si QDs as multifunctional probes for hydrophobic drug delivery and fluorescence-guided therapy.
The (3-Aminopropyl)trimethoxysilane (97%), Trisodium citrate dehydrate (≥ 99.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Silicon phthalocyanine dichloride (defined as Pc), LysoTracker Green DND-26 and DMSO were purchased from Sigma-Aldrich. All chemicals were used without additional purification. Milli-Q water (Millipore) was used as solvent for solution preparation. For the in vitro and in vivo tests, a stock solution of Pc in DMSO was diluted with cell culture medium or physiological saline to make sure that the cells were never exposed to more than 1% DMSO. Human gastric carcinoma cells (MGC-803) were purchased from the cell bank of the Chinese Academy of Sciences in Shanghai and cultured in Dulbecco’s Modified Eagle Medium (DMEM, Hyclone) with 10% FBS in a 5% CO2 atmosphere at 37 °C and 100% humidity.
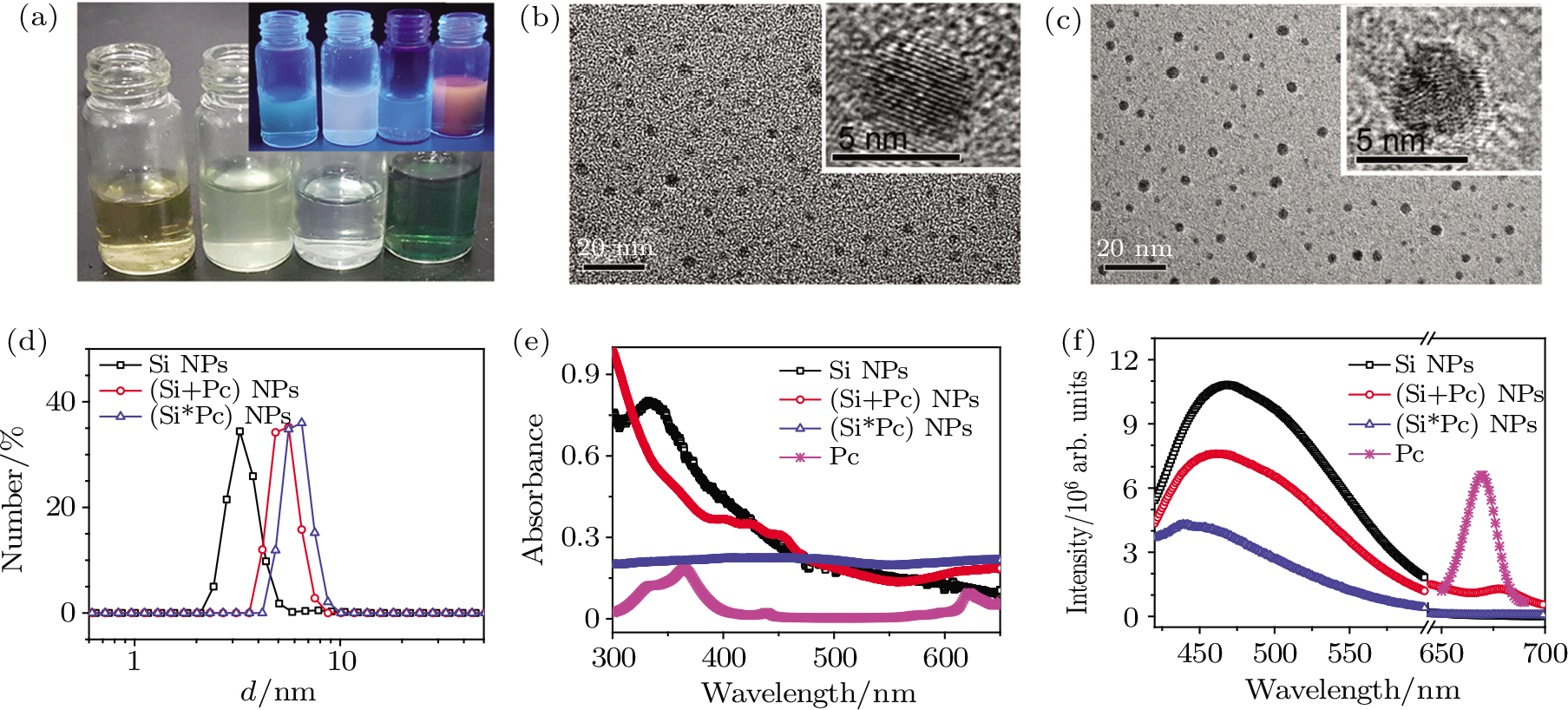
The pure Si QDs were firstly synthesized following our previous protocol.[12] In brief, 100 mL of (3-aminopropyl)trimethoxysilane was added into 400 mL of N2-saturated aqueous solution containing 18.6 g trisodium citrate dehydrate and stirred for 30 min. A 15 mL of the well mixed solution was transferred to a quartz vessel, heated to 160 °C by microwave irradiation in a microwave system (at 2450 MHz frequency and 0–500 W power; Preekem of Shanghai, China). The solution was incubated at 160 °C for 3 h, and then cooled down to room temperature naturally. The obtained solution was purified by dialysis (500 Da) to remove the excessive chemicals, and concentrated by a rotary evaporator. The obtained Si QD dispersion, at a concentration of around 7.2 mg⋅mL−1 based on the UV–vis absorption measurement, was taken as mother solution for the following use.
The Si QDs were loaded with Pc by two different methods, i.e., physical adsorption and chemical binding, and referred to as (Si+Pc) and (Si*Pc) NPs, respectively. 1) For the preparation of (Si+Pc) NPs, Pc was pre-dissolved in ethanol at 10 mg⋅mL−1 with the help of sonification. A 5 mL of the Si NP mother solution was added into 1 mL of Pc solution. The mixture was kept in the dark for 3 days with gently shaking. After that, the solution was thoroughly blown with nitrogen for fully evaporating the ethanol in the mixture and the adsorption occurred between Pc and Si via hydrophobic interaction.[19] The obtained solution was filtered through a 0.45 μm filter four times to remove the excessive Pc. The final concentration was determined to be around 6.58 mg⋅mL−1 (in respect to Si) and 1.0 mg⋅mL−1 (in respect to Pc), with a Pc-loading capacity of ~152 mg⋅g−1.
2) On the other hand, for the preparation of (Si*Pc) NPs, Pc was pre-dissolved in DMSO at 10 mg⋅mL−1 by sonification. A 10 mL Si QD mother solution was dropped into 4.6 mL Pc solution slowly. The mixture was sealed, heated to 90 °C, and kept for 72 h with magnetic stirring. The amino-group on the Si QD surface is supposed to be substituted for silicon-chlorine bond with Pc in this process.[30] The solution was cooled down to room temperature naturally, purified by dialysis (500 Da) to remove the excessive chemicals, and concentrated by a rotary evaporator. The final concentration was determined to be around 9.87 mg⋅mL−1 (in respect to Si) and 1.4 mg⋅mL−1 (in respect to Pc), with a Pc-loading capacity of ~ 142 mg⋅g−1.
The Si, (Si+Pc), and (Si*Pc) NP dispersions were observed in ambient light and UV irradiation (λex = 365 nm) separately. The NPs were characterized with transmission electron microscopy (TEM; CM 200, Philips), dynamic light scattering (DLS; DynaPro, Malvern), x-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, Thermo), zeta potential (Zetasizer Nano ZS90, Malvern), UV–vis absorption (UV3600, Shimadzu) and photoluminescence (PL; FluoroMax-4, Horiba JobinYvon). A confocal laser scanning fluorescence microscope (LSM 710, Zeiss), equipped with an in situ cell-incubation system and a 63× oil objective, was used for fluorescence imaging. Signals from the Si channel (EX 405 nm, EM BP 445/50 nm, excited by 15% power of a diode laser), Pc channel (EX 633 nm, EM 635–750 nm, excited by 30% power of a He–Ne laser), and transmission channel (illuminated with a halogen lamp), were captured simultaneously. LysoTracker Green DND-26 was irradiated and collected in the green channel (EX 488 nm, EM BP 530/50 nm, excited by 20% power of an argon laser). All images were captured under the same instrumental settings and analyzed with image analysis software.
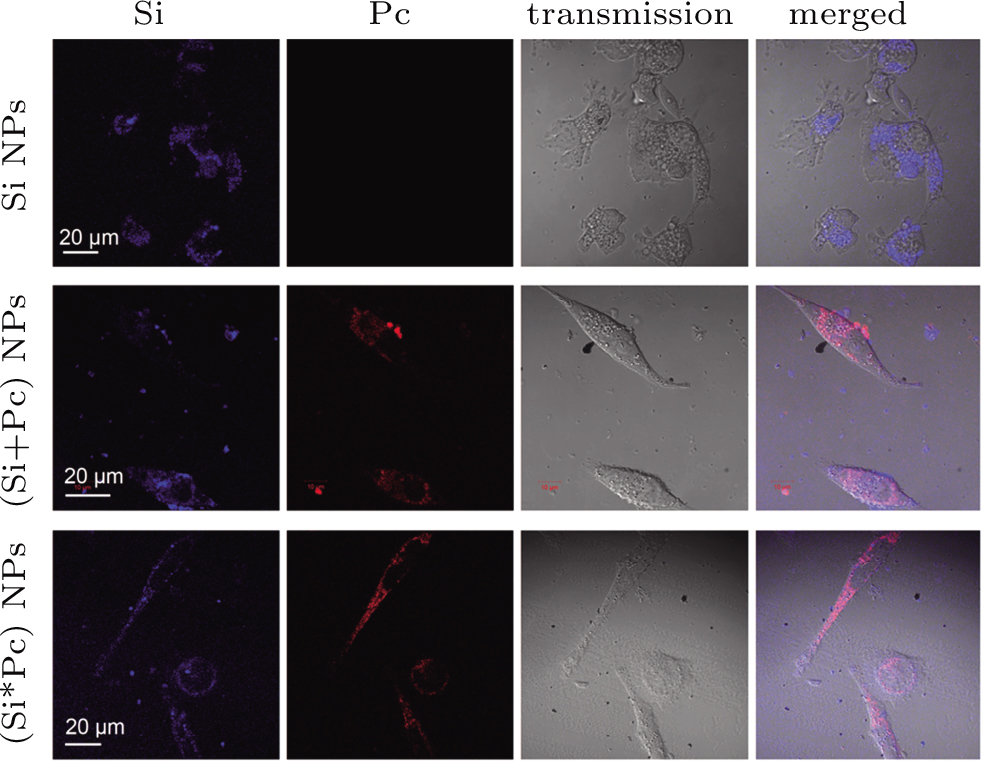
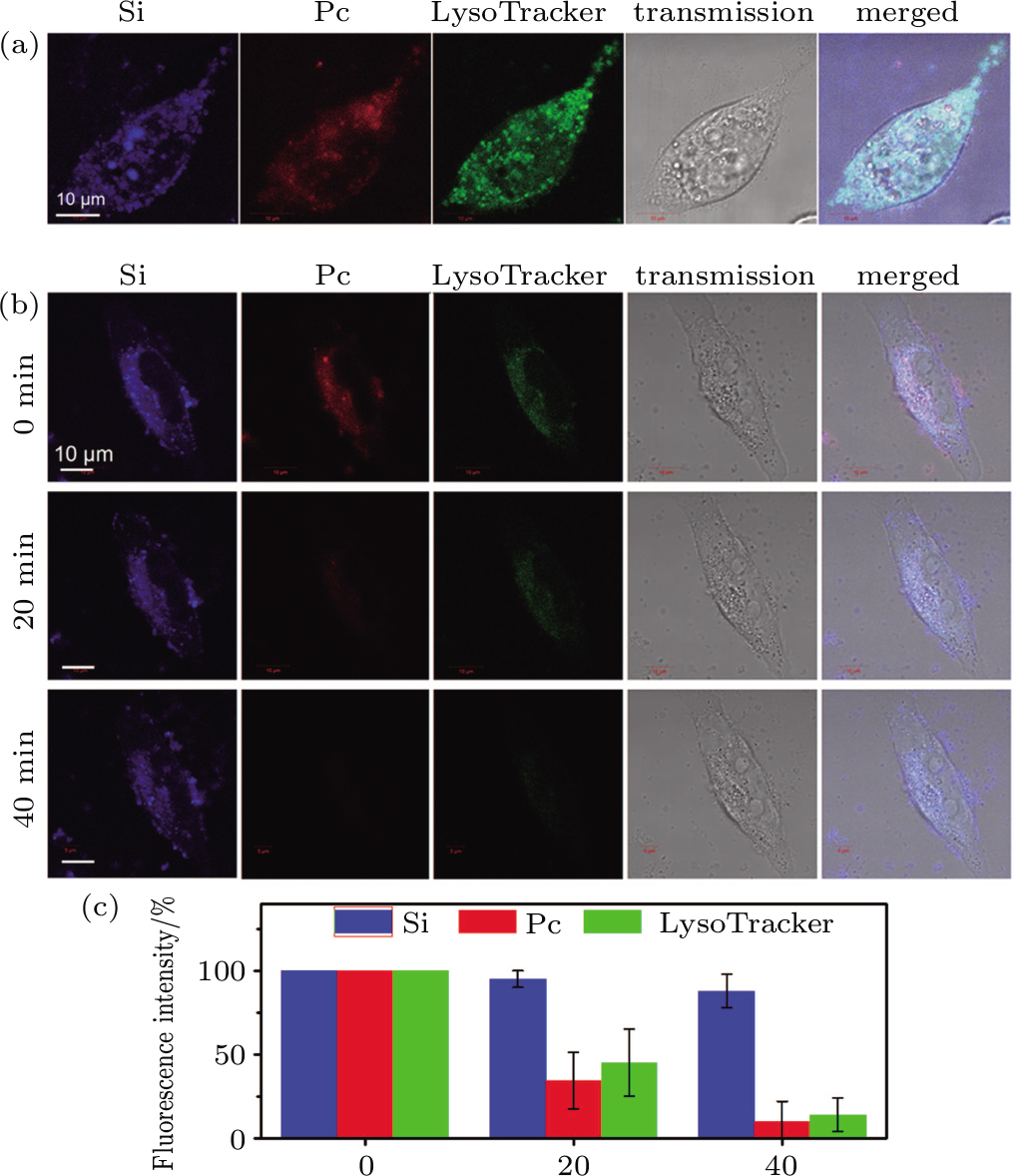
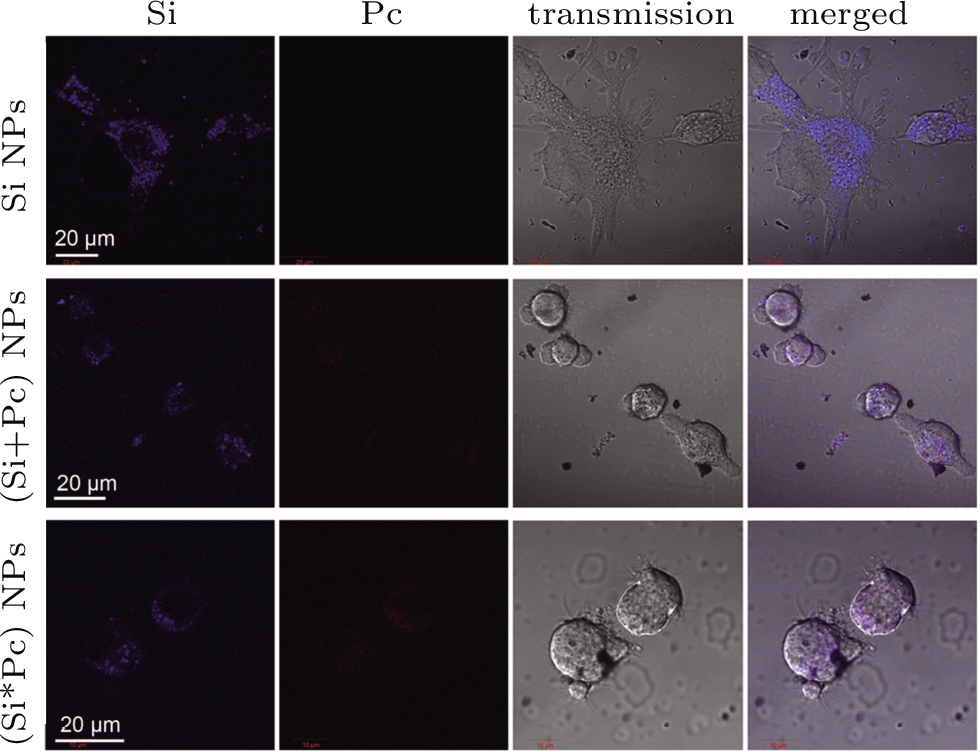
For the cellular uptake test, MGC-803 cells were pre-seeded on the cover glass substrate of a home-made unit (2 × 104 cells per unit). A certain amount of (Si+Pc), (Si*Pc) or pure Si NP dispersion was pre-diluted with cell culture medium to the same volume and added into the unit. The system (with a final volume of 500 μL) was further incubated for a certain time duration as described in the main text. Prior to the observation, the cells were washed with phosphate buffer saline (PBS) 3 times to remove the free NPs and re-cultured with fresh medium. To determine the intracellular localization of NPs, the cells, after NP co-incubation for 24 h, were further treated with the LysoTracker Green DND-26 (at 100 nM for 30 min), and washed prior to the confocal observation.
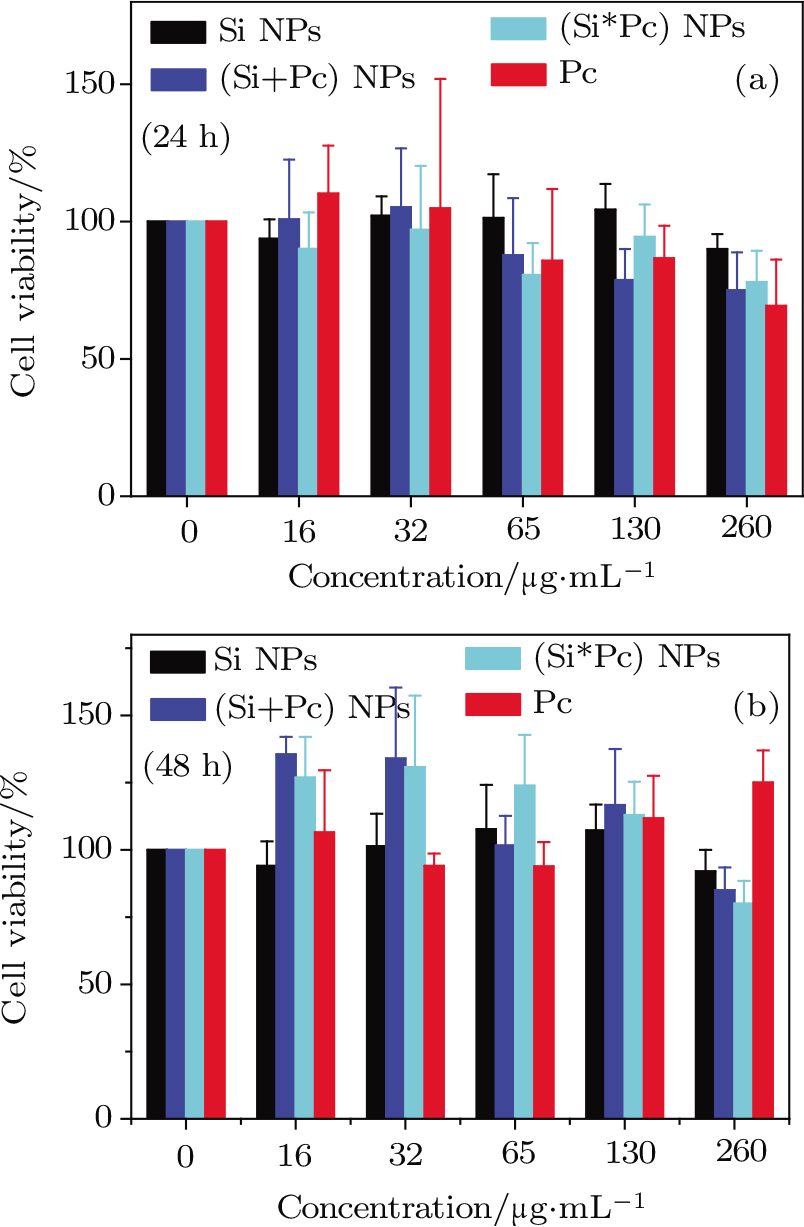
For the cytotoxicity test of the NPs, MGC-803 cells were pre-seeded in a 96-well plate (3000 cells per well), then co-incubated with the (Si+Pc), (Si*Pc), pure Si NPs or free Pc at a certain concentration (from 0 to 260 μg⋅mL−1 in respect to Si or from 0 to 40 μg⋅mL−1 for free Pc; this ratio was selected based on the loading amount of Pc within the (Si+Pc) NPs) for 24 h or 48 h. The relative number of the viable cells was then evaluated by the standard MTT assay.
Photostability evaluation of the NPs was carried out in situ under the confocal microscope, on the cells which have been internalized with NPs and labeled with LysoTracker. The (Si+Pc) NP system was taken for example. The cells were exposed to a 488 nm irradiation by the argon laser (at 2% power) for different time intervals. Confocal images, in all the Si, Pc, and LysoTracker channels, were captured after each irradiation interval (e.g., 0, 20 min, and 40 min) with identical instrumental settings for comparison.
For the in vitro PDT test, the cells, which have been co-incubated with the pure Si, (Si+Pc) or (Si*Pc) NPs for 24 h, were exposed to an NIR irradiation at 610 nm wavelength and 25 mW⋅cm−2 power for 30 min followed by PBS washing and fresh medium replacing. After that, the morphology of the cells was observed under confocal microscope and cell viability was evaluated with the established MTT assay.
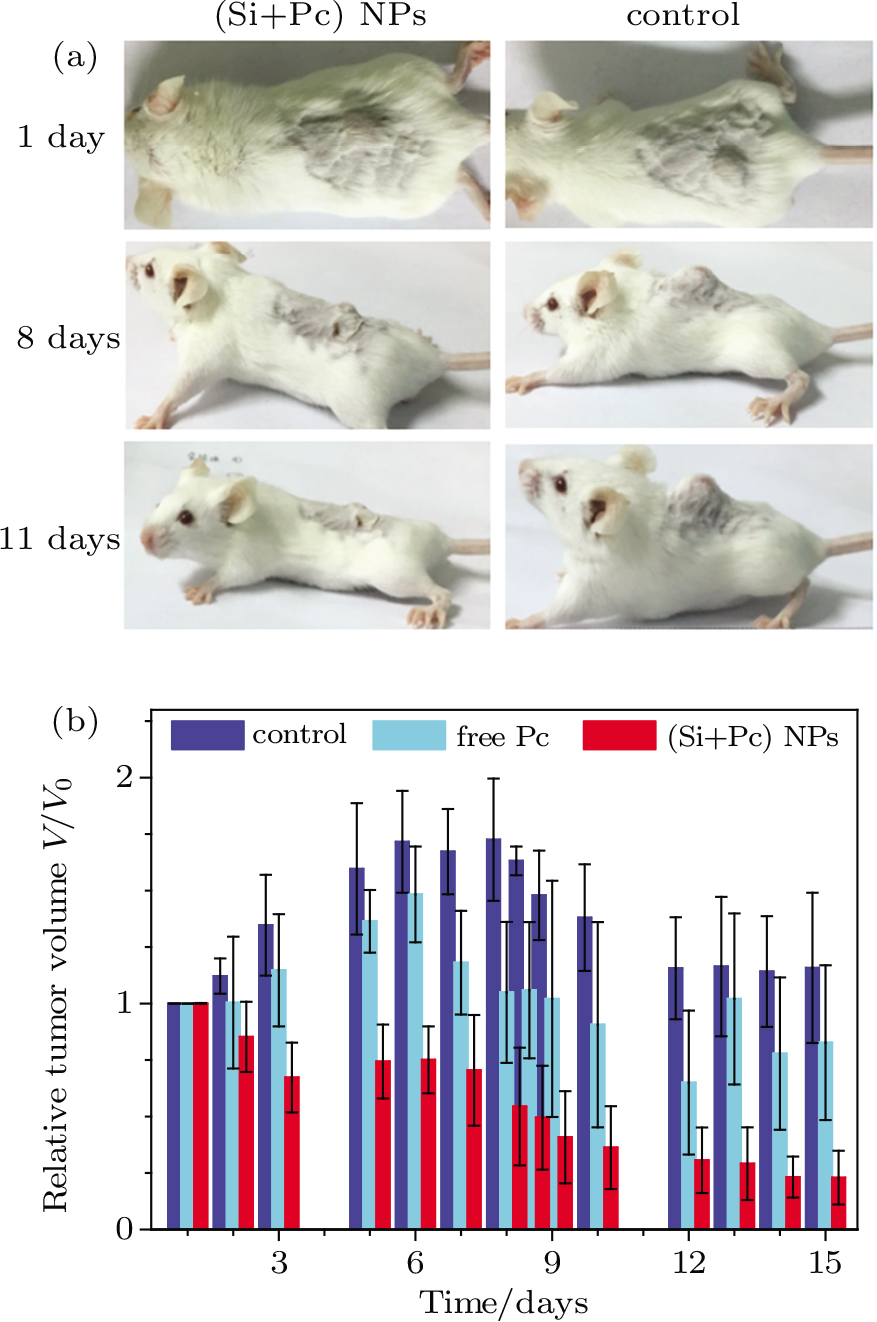
MGC-803 tumor bearing mice were intratumorally injected with 150 μL of (Si+Pc) NP dispersion (pre-diluted with physiological saline) on the 1st day and 7th day. Physiological saline or free Pc (pre-diluted with physiological saline) with the same volume was taken as a control. The dose of Pc content was fixed at 7.5 mg⋅kg−1 (or might be lower than this value for the free Pc system due to the obvious precipitation of Pc in PBS). Four hours after each injection, the tumor area was irradiated by the 610 nm laser at 25 mW⋅cm−2 for 30 min. The tumor sizes were measured every the other day and the tumor volume V was calculated as V = (tumor length) × (tumor width)2)/2.[31] The relative tumor volumes were normalized based on the initial value on the 1st day.
The hydrophobic drug Pc was loaded to the Si NPs through physical adsorption (named (Si+Pc) NPs) and chemical binding (named (Si*Pc) NPs) respectively. The as-obtained composite NPs in addition to the initial Si NP dispersions, were collected and characterized as shown in Fig.
The Si, (Si+Pc) and (Si*Pc) NPs are co-incubated with MGC-803 cells respectively, for different time durations prior to observation under confocal microscope. The time-dependent transmembrane entry of the nanocomposite NPs was observed (Fig.
The fluorescence stability of the internalized NPs is further confirmed by continuous laser exposure in situ. The fluorescence intensities in the Si, Pc and commercial LysoTracker channels are analyzed respectively after irradiation of different time durations, both qualitatively and quantitatively (Figs.
The cells with internalized NPs show regular morphology which indicates little cytotoxicy of the particles. Quantitative cellular viability analysis after NP treatment for 24 h or 48 h at various NP concentrations from 0 to 260 μg⋅mL−1 in respect to Si content (or from 0 to 40 μg⋅mL−1 for free Pc), based on the standard MTT test, is shown in Fig.
The Pc is a representative type of PDT drug which can generate reactive oxygen species (ROS) under laser irradiation at a certain wavelength to kill tumor cells. Here, the MGC-803 cells with internalized Si, (Si+Pc), and (Si*Pc) NPs, are irradiated by a laser (at 610 nm with a power of 25 mW⋅cm−2) for 30 min and the resulting cells are observed under confocal microscope or analyzed with standard MTT test. The representative images of the cells are shown in Fig.
Mice bearing MGC-803 tumor at their back are used as models to testify the in vivo anti-tumor effect of the Si NP-based nanocomposite drugs. Representative images of the mouse are shown in Fig.
In this work, we produce a type of water-dispersible Si QD, and the hydrophobic drug Pc is efficiently loaded on the Si QDs via facile preparation such as physical adsorption or chemical conjugation. The as-obtained Si/Pc nanocomposite particles are well water-dispersible and have small sizes below 5 nm. They can be effectively uptaken by cells while show feasible cytotoxicity, and maintain stable even after cell internalization. The composite particles are imaged by dual-channel fluorescence signals in Si and Pc channels. We find that the fluorescence in the Si channel shows robust stability promising its applications for long-time tracking and bioimaging. More interestingly, the composite nanoparticles demonstrate significant PDT anti-tumor effects both in vitro and in vivo. Based on the Si QDs, these results provide a novel strategy for the efficient delivery of hydrophobic drugs and their use for fluorescence imaging guided photo dynamic therapy against tumors.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] |