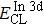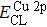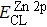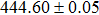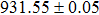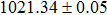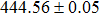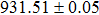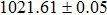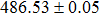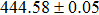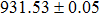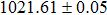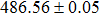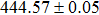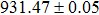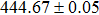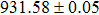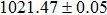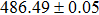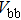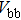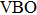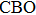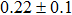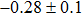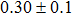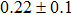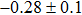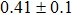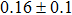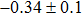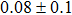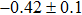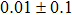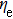† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 61574038 and 61674038) and the Natural Science Foundation of Fujian Province, China (Grant No. 2014J05073).
The effect of the deposition temperature of the buffer layer In2S3 on the band alignment of CZTS/In2S3 heterostructures and the solar cell performance have been investigated. The In2S3 films are prepared by thermal evaporation method at temperatures of 30, 100, 150, and 200 °C, respectively. By using x-ray photoelectron spectroscopy (XPS), the valence band offsets (VBO) are determined to be 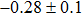
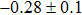
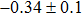
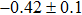
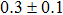
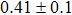
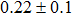
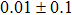
The Cu2ZnSnS4 (CZTS) thin film is one of the most promising light absorbing materials which has attracted much research interests recently.[1–4] In highly efficient CZTS solar cells, CdS is usually utilized as a buffer layer.[4–6] However, since Cd is toxic, great effort has been devoted to developing CZTS solar cells with Cd-free buffer layers.[5–7] Being non-toxic, In2S3 is a preferable material to replace CdS as a buffer layer.[5,6] Since conduction band alignment between the p-type CZTS and n-type buffer layers is a key factor to influence the device performance, it is of great importance to investigate the band offset of CZTS/In2S3 heterostructures.[6] Yan et al. investigated the band alignment of different buffer layers, i.e., CdS, Zn(O,S) and In2S3 on CZTS, and they found that the conduction band offset (CBO) of In2S3/CZTS is spike-like with a value of 
In the experiments, four CZTS/In2S3 heterostructure samples were prepared. The CZTS thin films with a thickness of 800 nm were fabricated on floating glass (FG) substrates by sol–gel method, followed by sulphurization in an N2 + H2S gas atmosphere (with 5% H2S concentration) at 580 °C for one hour.[10] The buffer layers In2S3 with a thickness of 5 nm were deposited onto the CZTS thin films by thermal evaporation method in DMDE-450 deposition equipment. The substrate temperature during the deposition of In2S3 was chosen to be 30 (room temperature), 100, 150, and 200 °C, respectively. In order to make the deposition of such a thin film possible, the substrate and the source material was kept at a far distance of about 22 cm. In order to find out the right amount of source material (In2S3 powder) to deposit an In2S3 film of 5 nm, 30-, 50-, and 100-mg In2S3 powder were used as the source material, respectively, and the corresponding thicknesses of the In2S3 films were determined to be 14, 25, and 49 nm by a step profiler (KLA Tencor D-100). Therefore, to deposit an In2S3 film with a thickness of 5 nm, 5-mg In2S3 powder was used as the source material. Besides, another four In2S3 films with a thickness of 400 nm were also prepared by thermal evaporation method, which were deposited on FG substrates at the four temperatures mentioned above, respectively. The transmittance and reflectance spectra were measured by a Cary 5000-Scan UV-vis-NIR Spectrometer in the wavelength range 350 nm–1200 nm. The XPS measurements were carried out using a ThermoFisher Scientific Escalab 250 electron spectrometer with monochromatic Al Kα (
To fabricate a complete solar cell, an intrinsic ZnO film of 60 nm and an ITO film of 270 nm were deposited on top of the glass/Mo/CZTS/In2S3 (50 nm) stack by sputtering. Then, 100-nm Al metal grids were fabricated by thermal evaporation. The J–V curves of the fabricated CZTS solar cells with the buffer layer In2S3 deposited at different temperatures were measured by a solar simulator with an illumination intensity of 100 mW/cm2 (Oriel 91192, AM1.5, Global).
According to Ref. [11], the valance band offset (VBO) can be calculated using the formula
 |
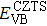
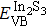
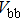
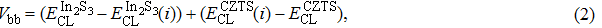 |

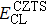
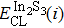
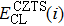
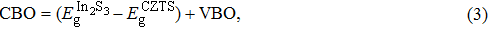 |
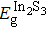
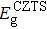
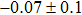
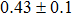
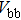
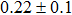
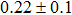
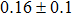

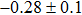
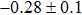
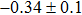
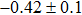

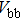
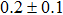
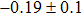
| Table 1.
Core-level energies obtained by the XPS spectra fitting for the bulk In2S3, bulk CZTS, and CZTS/In2S3 heterostructures deposited at different temperatures. . |
| Table 2.
|
The band gap 
 |
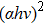
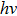
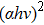
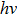
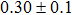
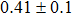
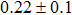
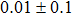
The CBO can also be easily calculated by using the Anderson model,[12] if electron affinity χ of In2S3 and CZTS are known. Electron affinity χ of In2S3 and CZTS are estimated to be 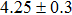
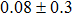
With the values of VBO, CBO, and 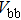
| Table 3.
Element content at the interface of the heterostructures deposited at different temperatures. . |
In order to investigate the influence of the deposition temperature of the buffer layer as well as the band alignments of the heterostructures on the device performance, the CZTS solar cells with the buffer In2S3 deposited at temperatures of 30, 100, 150, and 200 °C, named as samples A, B, C, and D, respectively, are prepared. J–V curves of the devices are shown in Fig. 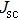
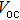
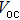
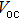
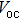
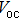

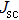
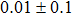
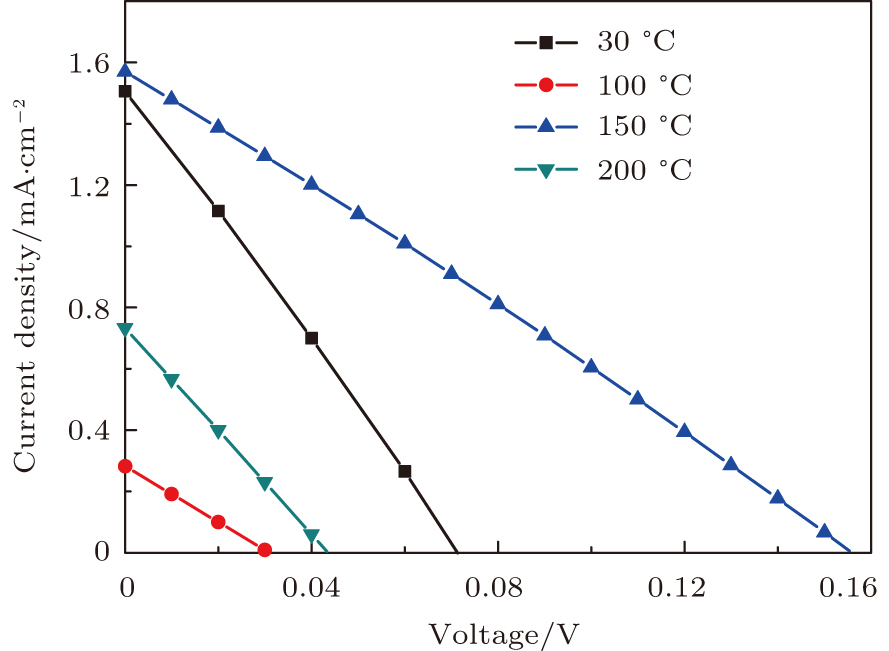 | Fig. 3. (color online) J–V measurements of the solar cells with the buffer layer deposited at different temperatures under AM1.5 solar illumination conditions. |
| Table 4.
Performance characteristics of the solar cells with the buffer layer deposited at 30 (room temperature), 100, 150, and 200 °C, respectively. . |
In summary, we have studied the influence of the deposition temperature of the buffer layer on the band alignment of CZTS/In2S3 heterostructures. It is found that the VBO values are 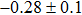
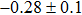
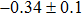
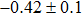
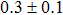
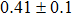

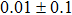
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] |