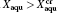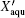† Corresponding author. E-mail:
Project supported by the Knowledge Innovation Project of Chinese Academy of Sciences on Water Science Research (Grant No. KJZD-EW-M03) and the National Natural Science Foundation of China (Grant Nos. 11474325 and 11290161).
For aqueous solutions with freezable bound water, vitrification and recrystallization are mingled, which brings difficulty to application and misleads the interpretation of relevant experiments. Here, we report a quantification scheme for the freezable bound water based on the water-content dependence of glass transition temperature, by which also the concentration range for the solutions that may undergo recrystallization finds a clear definition. Furthermore, we find that depending on the amount of the freezable bound water, different temperature protocols should be devised to achieve a complete recrystallization. Our results may be helpful for understanding the dynamics of supercooled aqueous solutions and for improving their manipulation in various industries.
Recrystallization, or cold crystallization, refers to the crystallization process invoked by heating the devitrified liquids, which is a crucial phenomenon that can be observed in cryopreserved foods and living organs,[1–4] polymers,[5–8] metallic glass-forming alloys,[9–12] and aqueous solutions.[13–16] Generally speaking, the recrystallization process can be categorized into two distinct classes according to whether the devitrified liquids totally recrystallize or not. In one case, the devitrified liquid can totally recrystallize; therefore the recrystallized phase has the same composition as the devitrified liquid, as observed in most metallic glasses[10–12,17] and polymers.[6,18–20] In the other case, the devitrified liquid recrystallizes incompletely, and the residual phase maintains the liquid status upon further heating, as widely observed in aqueous solutions[13,21,22] in a particular concentration range depending on the nature of the solute.
The recrystallization process in aqueous solutions has already been investigated by using differential scanning calorimetry (DSC)[13,21–24] and spectroscopic techniques such as x-ray diffraction,[25] nuclear magnetic resonance,[26] and mid-infrared (MIR).[16,27,28] The water molecules within such solutions have been classified into two types. Those water molecules that vitrify upon cooling but can be set free to recrystallize upon reheating have been termed freezing bound water[29] or intermediate water.[28] The other portion of water that can easily vitrify with the solute but cannot recrystallize upon the subsequent reheating process is usually called non-freezing bound water.[29,30] For both scientific and technical purposes, it is essentially important to find a convincing and reliable scheme to exactly quantify these two kinds of water, which we prefer to term them as freezable bound water and non-freezable bound water. In the past, the amount of freezable bound water is generally deduced from the melting enthalpy of precipitated ice, ΔHice, obtained by integrating the endothermic peak on the DSC heating curve, to which the value of the melting enthalpy for bulk ice (334 J·g−1) is used in conversion. Regretfully, the assumption that ΔHice is independent of the solution concentration and/or the temperature may introduce a large error.
The recrystallization of the freezable bound water leaves behind a residual solution which comprises solute and the non-freezable bound water, which is thus referred as the freeze-concentrated phase. Therefore, the amount of non-freezable bound water can be determined so long as the concentration of the freeze-concentrated solution has been fixed. Traditionally, this concentration is viewed as corresponding to the point at which the extrapolated equilibrium liquidus, i.e., Tm versus concentration curve, for precipitated ice and the glass–liquid transition temperature, Tg, versus concentration curve for solute-rich solutions intersect (Fig. 23 in Ref. [31]). This quantification scheme is applicable to solutions of large organic molecules with a narrow gap between the equilibrium liquidus curve and the Tg versus concentration curve.[13] Recently, it is suggested that equilibrium liquidus should not be exrapolated so far to come across the Tg versus concentration curve due to the broader range of temperature for maximum ice formation (Fig. 


In the current article, we report a simple scheme for the precise quantification of freezable bound water involving only the water-content dependence of glass transition temperature, Tg. It will make clear the point that the non-freezable bound water is in fact the hydrated water that characterizes the hydration ability of a given solute. Ice recrystallization only occurs in a solution containing more water than specified by the hydration water, but only up to a critical threshold beyond which spontaneous crystallization occurs during the cooling process. Namely, the aqueous solutions that may undergo recrystallization, in a sense, fall within the medium-concentration range. The quantification scheme, and also the temperature protocol to provoke ice recrystallization in solutions of different amounts of freezable bound water, will be discussed on the basis of DSC and Raman spectroscopic data.
Various aqueous solutions in as large as possible range of concentration, specified by the mass fraction of water therein, Xaqu, were prepared with Millipore water and high-purity solutes ZnCl2 (99.99%), AlCl3·6H2O (99%), glycerol (99.5%), ethylene glycol (99.8%), sorbitol (99.5%), 1,2,4-butanetriol (98%), and polyethylene glycols 300 (PEG 300, Bioultra); all these are Sigma–Aldrich products. The DSC measurements were performed on a calorimeter (PE DSC8000) at a cooling/heating rate of 20 K/min. When cooled down to 123 K, the sample would be held at that temperature for 1 min before the heating procedure started. In this text, following the conventional practice,[33,34] glass transition temperature Tg was extracted from the onset point of the heating curve. During Raman spectra measurements, the temperature was adjusted within ±0.1 K using a cooling unit (Linkam L-600A) equipped with a temperature controller (Linkam TMS 94). Raman spectra were measured on a confocal Raman system (Jobin–Yvon HR800) with the 532-nm diode laser excitation. The laser power of 1 mW was focused onto the sample surface through a fused SiO2 film of thickness 0.3 mm. The integration time was set to 40 ms per point with a spectral resolution of 1.4 cm−1.
Figure 

The salient feature of the curves in Fig. 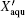
First, two different protocols for the cooling/heating process in DSC measurements were applied to prepare the solution in different status, and Raman spectra were recorded to reveal the chemical nature of the recrystallized phase induced by annealing treatment. Taking the aqueous ZnCl2 solution with Xaqu = 0.61 as an example, this solution totally vitrifies at Tg = 155.1 K (see Fig.
Next, to further reveal the chemical nature of the recrystallized phase, we check the water-content, Xaqu, dependence of Tg data for the aqueous ZnCl2 solution in the whole concentration range available (Fig. 






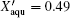



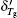

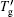

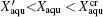

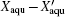
After recrystallization, the solutions of medium concentration at this stage comprise a mixture of pure ice and the freeze-concentrated solution with 

Based on the aforementioned results, some insights over the recrystallization of freezable bound water can be drawn. Freezable bound water is a concept in between the bound water contributing to hydration and free water which can easily crystallize spontaneously during the cooling process. It can vitrify together with the hydrated solute ions while recrystallize in the devitrified solution upon the continuously heating process or subjected to annealing treatment at temperatures between Tg and the recrystallization temperature. Of course, freezable bound water can also crystallize together with free water in the water-rich solutions upon cooling. Depending on the amount available, the characteristic nature of the freezable bound water can be more prone to behave as either the bound water or the free water, thus recrystallization of the freezable bound water in the medium-concentration solutions can only be observed with properly chosen cooling/heating protocols.
Lastly, we want to emphasize that the method for quantifying the freezable and non-freezable bound water in the current work can be applied not only to systems illustrated in Fig.
In summary, DSC measurement and Raman spectroscopy were employed to record the general features of recrystallization in aqueous solutions of several electrolytes, organic molecules, and their mixtures. Our results clearly revealed that ice recrystallization is a phenomenon to be anticipated in the aqueous solutions in a particular concentration range, and it involves only the freezable bound water. For a solution of medium concentration with a water content slightly larger than that characterized by the hydration formula, a deliberately designed temperature protocol is needed to provoke the ice recrystallization. A quantification scheme to determine the amount of the freezable bound water is provided based on the water-content dependence of glass-transition temperature, which can be exactly established by experiment. Our results have gained some insights into the dynamics of supercooled aqueous solutions; they may be helpful for improving the manipulation of supercooled solutions in various industries.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 |