† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11404133 and 11374121) and the Program of Science and Technology Development Plan of Jilin Province, China (Grant No. 20140520105JH).
Interface and scale effects are the two most important factors which strongly affect the structure and the properties of nano-/micro-crystals under pressure. We conduct an experiment under high pressure in situ alternating current impedance to elucidate the effects of interface on the structure and electrical transport behavior of two ZnSe samples with different sizes obtained by physical grinding. The results show that (i) two different-sized ZnSe samples undergo the same phase transitions from zinc blend to cinnabar-type phase and then to rock salt phase; (ii) the structural transition pressure of the 859-nm ZnSe sample is higher than that of the sample of 478 nm, which indicates the strong scale effect. The pressure induced boundary resistance change is obtained by fitting the impedance spectrum, which shows that the boundary conduction dominates the electrical transport behavior of ZnSe in the whole experimental pressure range. By comparing the impedance spectra of two different-sized ZnSe samples at high pressure, we find that the resistance of the 478-nm ZnSe sample is lower than that of the 859-nm sample, which illustrates that the sample with smaller particle size has more defects which are due to physical grinding.
Using a mature technique to synthesize the same material with different morphologies and grain sizes, scientists are able to accurately control the properties of materials through changing the experimental conditions.[1–4] Different morphologies or grain sizes may differ in both chemical and physical properties even for the same sample with the same structure, which is due to the different arrangements of surface atoms and the ratio of the number of atoms on the surface to that in the bulk of nano-/micro-structured crystals, corresponding to the ratio of the surface energy and the cohesive energy.[4–6]
Pressure can induce direct and significant changes in atomic and electronic structure, and allows the tuning of the properties. In some earlier publications, the size effects in ZnS,[7] ZnO,[8] AlN,[8] Y2O3,[9] etc. under high pressure have been reported. These reports showed that the sample properties under high pressure are highly dependent on the initial grain size synthesized by the chemical method. The synthesis- and pressure-induced interfaces are named the chemical interface and physical interfaces, respectively. Since 2010, Wu et al. have reported that stress-induced silver nanoparticles turn into one-dimensional (1D)-nanostructure arrays,[10] a pressure-driven sintering of spherical nanoparticle arrays into nanostructured gold architectures,[11] and a pressure-induced silver nanoparticle super-lattice into mechanically stable nanowires.[12] Since 2012, the pressure-induced surface nanocrystallizations in Cu2O,[13] Alq3,[14] and Zn2SnO4 have been reported.[15] All the reported results in these two groups indicate that the pressure-induced physical interfaces affect the mechanical, optical, and electrical transportation properties, the phase sequence, transition pressure, etc.
As is well known, it is difficult to characterize the surface or interface properties directly under high pressure due to experimental restrictions. The researchers investigated the surface and interface properties by an indirect method through combining the SEM or TEM with the in situ optical measurements and in situ electrical transportation methods. To the best of our knowledge, there have been no in situ experimental data available under high pressure for the primitive samples with different surfaces and interfaces formed by the physical forces. Considering the different influences of chemical interfaces and physical interfaces, it will thus be valuable to conduct high pressure in situ experiments on the samples with different physical interfaces. The grind method is used to generate samples with different average grain sizes.
As a typical II–VI group semiconductor, Zinc selenide (ZnSe) has several potential applications and has been widely studied.[16–20] It was found that ZnSe has size-dependent optical and morphological properties,[21,22] and under compression the ZnSe transforms from zinc blende to cinnabar-type structure and then to rock salt structure.[23–27] Owing to the crystal size reduction, Campos et al. observed an increase of the phase-transition pressure.[28] However, it is not clear how the grain boundary changes and affects the electrical property of ZnSe particles under high pressure. In this paper, two ZnSe samples with different particle sizes obtained by the grind method and their grain boundary behaviors under high pressure are studied by in situ alternating the current (AC) impedance method.
The ZnSe sample with a purity of 99.999% was bought from Alfa Aesar Co. To obtain a different average size, the sample was ground manually by using an agate mortar. A Zetasizer nano series particle size analyzer was used to measure the particle size. Two samples with average sizes of 859 nm and 478 nm were obtained after one-and-two-hour grinding, respectively. In situ AC impedance spectrum measurements under high pressure with a two-electrode configuration microcircuit were performed in a diamond anvil cell (DAC) with an anvil culet of 400 μm in diameter. A T301 stainless steel gasket served as one electrode and a thin-film electrode integrated onto one diamond anvil surface served as another electrode.[29] More details about the microcircuit fabrication and the insulation between the gasket and the film electrode have been previously described.[30,31] The pressure was determined by the pressure induced shift of the R1 ruby fluorescence line.[32] No pressure medium was used to assure that the impedance spectrum reflects the information of the pure ZnSe samples. The AC impedance spectra were measured with a Solartron 1260 impedance analyzer equipped with a Solartron 1296 dielectric interface. An AC sine voltage signal with an amplitude of 1 V and a range from 0.01 Hz to 10 MHz was applied to the samples.
The impedance data of the ZnSe sample with a grain size of about 478 nm at different pressures are presented in Fig.
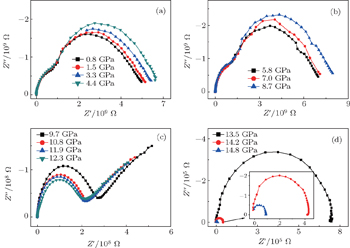 | Fig. 1. Complex impedance planes of ZnSe with an average grain size of about 478 nm at pressures of (a) 0.8 GPa–4.4 GPa, (b) 5.8 GPa–8.7 GPa, (c) 9.7 GPa–12.3 GPa, and (d) 13.5 GPa–14.8 GPa. |
As shown in Fig.
When the pressure rises to 13.5 GPa, the impedance spectrum transforms into only one semicircle representing the boundary conduction as depicted in Fig.
The impedance data of the ZnSe sample with a grain size of 859 nm at different pressures are shown in Fig.
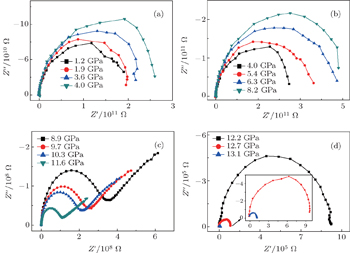 | Fig. 2. Complex impedance planes of ZnSe with an average grain size of about 859 nm at pressures of (a) 1.2 GPa–4.0 GPa, (b) 4.0 GPa–8.2 GPa, (c) 8.9 GPa–11.6 GPa, and (d) 12.2 GPa–13.1 GPa. |
When pressure is higher than 8.9 GPa (as shown in Fig.
Above 12.2 GPa (as shown in Fig.
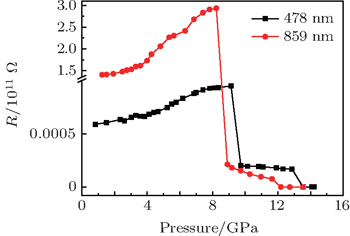
By comparing the impedance spectrum of 478 nm and that of the 859-nm ZnSe sample, we find that (i) the change trends of electrical properties of two specimens are consistent, and they undergo the same phase transition pathway from zinc blende to the cinnabar-type phase and then to the rock salt phase; (ii) the smaller grain size sample needs higher pressure points of structural phase transition; (iii) the boundary conduction plays a dominant role in the transportation process under high pressure. In order to observe the change of the grain boundary in a more intuitive manner, we obtain the resistance of the grain boundary by fitting the impedance spectra of two specimens, the results for the corresponding resistance are plotted in Fig.
Figure
As the pressure is increased, the grain boundary resistances of the two samples decrease sharply. This is due to the structure phase transition of the sample from zinc blende to cinnabar phase structure. The grain boundary resistance decreasing interval (between 8.0 GPa–10.0 GPa) of the 859-nm ZnSe sample has a 0.8-GPa hysteresis compared with that of sample 478 nm. This is because the smaller the grain size, the higher the phase transformation pressure point is. After structural phase transition, the resistances of the two samples are equal to about 2.0×108 Ω. Simultaneously, the carriers in the grain boundary are located in the charge depletion layer, which will not participate in the conduction process of the defect level. In a pressure range of 10.0 GPa–14.0 GPa, the grain boundary resistance of ZnSe decreases obviously, which is attributed to the structure transformation from cinnabar phase to rock salt structure followed by the grain boundary resistance decline in this pressure range.
By AC impedance spectrum measurements of different sizes of ZnSe samples under pressure, we find that the phase transition pathways of ZnSe with different particle sizes are the same, which transforms from sphalerite to cinnabar and then to the salt mine structure. However, the phase transition pressure point of the small size sample is higher than that of the big size one. This result can explain the difference between the transition pressure of cinnabar reported by Kobayashi et al. and that by Pellicer–Porres et al. The analysis of the fitting results of the grain boundary resistance indicates that the grain boundary resistance plays a leading role in the changing of the electrical properties caused by the phase transition. By comparing the impedance spectra of two different particle size ZnSe samples at high pressures, we find that the resistance of the 478-nm ZnSe sample is less than that of 859 nm. This phenomenon is in contrast to the universal law that the smaller the sample size, the greater the resistance is. This is because the interface of the sample treated by physical grinding has more defects than the chemical interface sample. The defect energy level makes the electron excited from the highest occupied valence band to the lowest unoccupied conduction band easier. As a result, more carriers will participate in the conduction process. If the particle size of the sample is smaller, there would be more defects, and the resistance of the grain boundary would be smaller. Therefore, when studying the electrical properties of a power sample, one should consider not only the scale effect but also the interface type.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 |