† Corresponding author. E-mail:
Project supported by the National Major Scientific and Technological Special Project during the Twelfth Five-year Plan Period of China (Grant No. 2009ZX02030-1), the National Natural Science Foundation of China (Grant No. 51205387), the Support by Science and Technology Commission of Shanghai City, China (Grant No. 11nm0500300), and the Science and Technology Commission of Shanghai City, China (Grant No. 14XD1425300).
Metal Ti and its alloys have been widely utilized in the fields of aviation, medical science, and micro-electro-mechanical systems, for its excellent specific strength, resistance to corrosion, and biological compatibility. As the application of Ti moves to the micro or nano scale, however, traditional methods of planarization have shown their short slabs. Thus, we introduce the method of chemical mechanical polishing (CMP) to provide a new way for the nano-scale planarization method of Ti alloys. We obtain a mirror-like surface, whose flatness is of nano-scale, via the CMP method. We test the basic mechanical behavior of Ti–6Al–4V (Ti64) in the CMP process, and optimize the composition of CMP slurry. Furthermore, the possible reactions that may take place in the CMP process have been studied by electrochemical methods combined with x-ray photoelectron spectroscopy (XPS). An equivalent circuit has been built to interpret the dynamic of oxidation. Finally, a model has been established to explain the synergy of chemical and mechanical effects in the CMP of Ti–6Al–4V.
The metal titanium (Ti) and its alloys have been utilized since the 1950s. Owing much to less density and much stronger specific strength than steel, titanium alloys are becoming more and more important as load-bearing materials especially in an extreme environment like aviation.[1–3] In medical science, titanium alloys work as man-made teeth and joint thanks to their excellent resistance to corrosion and biological compatibility.[4–12] The last decade has witnessed a flourishing of the introduction of titanium to microelectronics and micro-electro-mechanical systems (MEMS).[13–16]
The wide use of metal titanium and its alloys calls for an effective method to achieve surface planarization of the nano-scale. Many researchers have studied the relationship between surface roughness and the device performance of Ti alloys (especially Ti64).[17,18] These studies demonstrate that the device performance can be improved dramatically by a smoother surface. Traditionally, the planarization of metal Ti relies on mechanical polishing, chemical polishing, and electrochemical polishing. Mechanical polishing, an ancient metal process, has been used for thousands of years, while its machine accuracy cannot reach the nano-size and it often leaves surface defections on metal. Chemical and electrochemical polishing do well in machine accuracy, however, the chemical matters used in these processes (like cyanide) are always poisonous to people and the environment. Chemical mechanical polishing (CMP), an amazing combination of mechanical polishing and chemical polishing, shows its potential in nano-size metal planarization. Up to now, it is the only method to provide a global nano-scaled planarization.
In this study, we discuss the basic behavior in CMP process of Ti64 alloy, which is the most widely used Ti alloy.[19–21] The mechanical and chemical aspects are both involved in our study. Finally, we propose a possible mechanism in the CMP of Ti64, focusing on the rate controlling step in CMP.
Polishing slurries were prepared by first adding ethylenediaminetetraacetic acid disodium (EDTA-2Na) to deionized (DI) water until dissolved, immediately followed by the mixture with colloidal silica under successive stirring. EDTA-2Na was used as the chelating agent to capture free metal cations in solution so that the bulk concentration of metal cations could keep a low level. The colloidal silica has a diameter of about 110 nm (Fig.
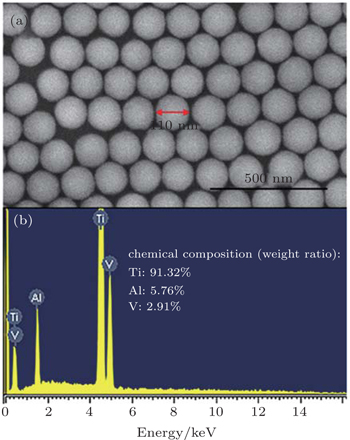 | Fig. 1. (a) The colloidal silica particles used as abrasive particles in this study; (b) the chemical composition measured by the EDS. |
| Table 1. The compositions of slurries used in the polishing experiments. . |
The Ti64 alloy was cut into circular pieces of 1 inch in radius. The element composition was analyzed using energy dispersive spectroscopy (EDS, see Fig.

To probe the possible oxidation mechanism of Ti64 during CMP, electrochemical impedance spectroscopy (EIS) combined with linear polarization (LP) was used. The measurement was accomplished by an Autolab electrochemical workstation, with a three-electrode cell. An Ag/AgCl (in 3M KCl) electrode worked as the reference electrode, and a piece of platinum foil (1.5 × 1.5 cm2) worked as the counter electrode. The exposed area of metal to electrolyte is confined within unit area. The EIS measurements were applied at open circuit potential (OCP) of Ti64. A sine wave (±5 mV around OCP) as the perturbation signal was put into the measured system and the output signal was recorded by the computer. The frequency of input signal varied from 1 kHz to 0.1 Hz. Nyquist plots as results were shown to obtain an equivalent circuit which simulated the dynamic model of oxidation. Following the EIS test, LP was applied to obtain the message of corrosion potential (Ecorr) and corrosion current (Icorr). The scan was from −0.4 to 0.4 V (around OCP), with the scan rate being 1 mV/s.
Finally, the XPS (Axis Ultra DLD) was applied to analyze the valence state of metal atom as a support evidence to deduce the possible chemical reaction during CMP and the composition of oxide film.
The mechanical response of Ti64 during the CMP is diagramed in Fig.
The linear mechanical response in CMP can be well described by Preston’s law, which is

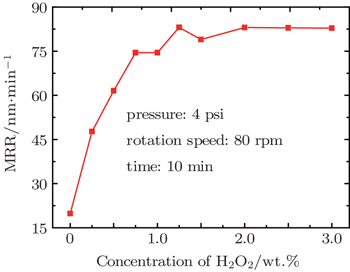
The chemical reactions are even more important in CMP than mechanical interaction. To have a basic understanding of the chemical mechanism in the CMP of Ti64, we restricted the possible reactions to be only the oxidation process and H2O2 was chosen as the oxidation agent. The effect of concentration of H2O2 on the MRR was studied, as shown in Fig.
It is obvious that H2O2 plays an important role in the CMP of Ti. When the slurry contains no oxidation agent, the MRR is neglectable. As H2O2 is added in the slurry, however, the MRR dramatically increases, and reaches its peak value of 83.05 nm/min when c[H2O2] is 1.25 wt.%. The curve shows a plateau when c[H2O2] is higher than 1.25 wt.%, which means that the oxidation effect has come to its maximum and there is an equilibrium between growth and removal of the oxide layer. Thus, we can assume that the oxidation layer is the main object removed by SiO2 particles during the CMP.
To probe the possible chemical reactions that possibly occur on the surface of Ti64 during the CMP, electrochemical tests were applied. The experimental details have been described in the experimental section. It is important to note that all the solutions under test are without abrasive particles so that the mechanical effects from those particles can be excluded. The other chemical properties of the solutions like the concentration of oxidant, pH, and ionic strength were adjusted to simulate the slurries used in the CMP. The EIS tests were performed under the OCP. For clarity, only five curves are illustrated (c[H2O2] = 0, 0.25 wt.%, 1 wt.%, 2 wt.%, and 3 wt.%). Before analyzing, we have applied K–K transform test on the data. As is clearly shown in Fig.
| Table 2. The values of each element in the equivalent circuit in Fig. |
It is obvious that the oxide layer acts as a constant-phase-element (CPE) more than an ideal plate capacitor. The α values in Table 2 are all around 0.92, which clearly shows the deviation. The CPE behavior is, according to Orazem,[26] generally attributed to a distribution of time constants in the direction normal to the electrode surface due to varying oxide composition. In terms of many other reports concerning the oxide films on titanium (for both metal and alloys), the passive film of this kind of valve metal appears as a very “capricious” formation. Different from the deemed impacted passive film consisting of pure TiO2, the real oxide film consists of two parts: a TiO2 layer adjoining solution and intermediate oxide layer (mainly composed of Ti2O3) close to the metal side.[27–31] It is easy to calculate the capacities in each concentration of H2O2 from the data in Table
 | Fig. 5. (a) The capacity of the oxide layer as a function of c[H2O2]; (b) the calculated thickness of the oxide layer as a function of c[H2O2]. |
The LP results are pictured as Fig.

(a) The Tafel curves of Ti64, with the scanning rate being 1 mV·s−1; (b) the corrosion potential and corrosion current deduced from the data above.
XPS can track the target element and show its valance state. Figure
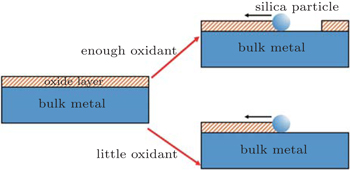
From the analysis above we can get a whole perspective of the CMP process of Ti64. Understanding the rate controlling step in CMP is the key point to combining the polishing results and electrochemical results together. The oxidation agent H2O2 plays an important role in CMP. It is the acceptor of electrons released by metal atoms, and leaves anamorphous, disorder, and nonstoichiometric oxide layer (Fig.
On the basis of the results above, we optimized the polishing parameters (4 psi, 80 rpm, and c[H2O2] is 1.25 wt.%) and applied them on Ti64. Figure
We have achieved a mirror-like surface of Ti64 by chemical mechanical polishing, which was in flatness of nano-scale. The CMP of Ti64 was studied in both mechanical and chemical aspects, and the parameters in the CMP process have been optimized. MRR increases with pressure, rotation speed, and the concentration of oxidant in slurry, and it can reach 100 nm/min. Meanwhile, the roughness was rather low. The chemical reactions during the CMP were studied by the electrochemical method. We have found that the thickness of the oxide layer is independent of c[H2O2] by utilizing EIS. The faster MRR is attributed to the fast reformation rate of oxide layer in enough concentration of H2O2 with the aid of LP. The composition of oxide layer is obtained by XPS, whose result coincides with EIS. Finally, we have proposed a possible model focusing on the rate controlling step during the CMP of Ti64.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 |