†Corresponding author. E-mail: diaojj@mail.xjtu.edu.cn
*Project supported by the Fundamental Research Funds for the Central Universities through Xi’an Jiaotong University and the National Key Basic Research Program of China (Grant No. 2015CB856304).
Among the techniques developed to prepare nanoparticle wires for multiple applications, the colloidal deposition method at interface has been regarded as cost-efficient and eco-friendly, and hence has attracted an increasing amount of research attention. In this report, the recent developments in preparing nanoparticle wires and integrated nanoparticle wire arrays using this technique have been reviewed. Furthermore, we have also discussed the application of these nanoparticle structures in detecting chemical and biological molecules.
Nanoparticles have garnered significant research interest owing to their unique electrical, thermal, and optical properties; significant research is being focused on the development of technologies for the applications of nanoparticles in the future.[1– 5] The development of techniques to assemble colloidal nanoparticles into functional structures, [6– 9] based on which nanosized devices could be built for potential nanotechnology applications, is an exciting research area.[10– 12] Many current research activities are devoted to the development of methods such as lithography for the fabrication of nanoparticle structures with good functional properties. These techniques either directly put nanoparticles in the shape of arrays [13, 14] or provide a platform for nanoparticle assembly.[15– 18] Although single-step assembly methods such as dielectrophoresis are very attractive, [19, 20] the control on the resulting structures is limited in these methods and careful tuning of the dielectric contrast between particles and solvents is necessary in order to achieve the best results.
Therefore, researchers have now resorted to the simple method of colloidal deposition to fabricate polycrystalline monolayer films and photonic structures at the air– liquid– solid interface.[21, 22] As shown in Fig. 1(a), evaporation of the solvent that suspends the colloidal nanoparticles will expose the solid substrate to air gradually. Due to interfacial forces, nanoparticles at the liquid– air– substrate interface deposit on the substrate along the meniscus. More details regarding this colloidal deposition at the air– liquid– solid for two-dimensional (2D) films can be found in recent review articles.[23– 25] Gold nanoparticle films produced using this method[26] serve as a good matrix for laser desorption ionization mass spectrometry.[27] Furthermore, this method is very generic and hence can be applied on a wide range of metallic and nonmetallic nanoparticles as well as different solvents and substrates.
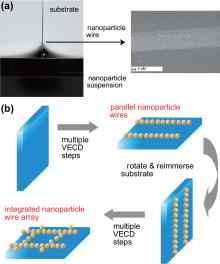 | Fig. 1. Illustration of colloidal deposition at the air– liquid– solid interface to produce nanoparticle wires. (a) The meniscus region of a vertical evaporation-driven colloidal deposition (VECD) system. When the deposition is uninterrupted, a continuous nanoparticle thin film is formed as a 2D nanoparticle structure. When the deposition is interrupted by rapid removal of a small quantity of the suspension, a 1D structure of nanoparticle wires is formed. The inset is a scanning electron microscopy (SEM) image of a gold nanoparticle wire made by discontinuous VECD. (b) Schematics of the process of building integrated nanoparticle wire arrays through a multi-step discontinuous VECD. Parallel nanoparticle wires and integrated nanoparticle wire arrays are formed after the first and the second deposition step, respectively.[47] |
Meanwhile, in the last decade, multidisciplinary approaches using nanotechnology and biotechnology techniques have gained popularity in the international research community.[28] Many researchers have shown that such fusion has the potential to revolutionize a host of industries in the future.[29– 32] Effective utilization of concerted applications has recently been exemplified by breakthroughs in bio-directed nano-synthesis/assembly and by the use of nanotechnology for biological detections. Further advancement in biomedicine requires new detection systems for disease biomarkers and therapeutic compounds.[33] Nanoparticle structures built using colloidal deposition are promising candidates for the sensitive detection of chemical and biological molecules.
While uninterrupted solvent evaporation results in a continuous deposition of nanoparticles to form a 2D film, discontinuous evaporation interrupted by rapid movement of the solution surface leads to the formation of a one-dimensional (1D) nanoparticle structure.[34– 41] Subsequent movement of the solution surface leads to a stepwise formation of nanoparticle wires on the substrate (Fig. 1(b)). Different ways to induce rapid movement of the solution surface such as quick removal of the solution, [35, 38] rapid rise of the substrate level, [36, 37, 40] and accelerated evaporation[34, 39, 40] have been used.
These nanoparticle wires are formed along their width, the magnitude of which is determined by the evaporation rate and the deposition time: speeds of hundred nanometers per second and times of several seconds to minutes.[42, 43] By using a thin 2D Langmuir– Blodgett film of nanoparticles, Huang et al. were able to produce chains of individual nanoparticles.[37] The length of these wires was limited only by the substrate size, which can easily reach centimeter levels. The distance between two adjacent wires can be conveniently controlled by controlling the distance travelled by the solution surface between each deposition step.[44]
Uniformly dispersed aggregations of nanoparticles on the contact line depend on the fingering instability during the initial de-wetting stage. As shown in Fig. 2, these fingertips then guide further deposition of nanoparticles, finally forming the extended striped pattern along their length.[45, 46]
 | Fig. 2. (a) Atomic force microscopy (AFM) and (b) scanning electron micrograph (SEM) images of nanoparticle wires produced by discontinuous VECD. (c) SEM image of parallel nanoparticle wires and (d) optical micrograph of nanoparticle wire array.[35] |
Once a layer of parallel wires is deposited (Fig. 1(b)), the substrate is removed from the suspension, rotated at an angle, and re-immersed. Following a second deposition process, another layer of parallel wires is deposited to form an integrated nanoparticle wire array (Fig. 2). The angle between two wire layers in the array depends on the rotation angle of the substrate between the two deposition steps.[37, 41, 47, 48] This multi-step deposition strategy can also be applied to build double-component nanoparticles wire arrays by switching to a different nanoparticle suspension during the second deposition step.[41, 47, 48]
As the conductance of gold can be influenced by its surrounding environment, [49] the behavior of nanowires can also be affected by chemical and biological molecules such as proteins and viruses.[50, 51] Because of a high surface-to-volume ratio of the porous nanoparticle structure, the gold wires produced using the colloidal deposition are promising candidates for use in the sensitive electronic detection of biological and chemical objects (Fig. 3). For thiol molecules, the increase in the resistance of a gold nanoparticle wire is about 10 times larger than that obtained using the thiol-modified gold thin film.[52] Compared to porous gold nanowires produced by in situ etching, [53] these gold nanoparticle wires show a 3-fold higher sensitivity. Due to the advantage of high surface to volume ratio of these wires, we were able to achieve sensitivity high enough to detect DNA molecules at a concentration as low as 1 pM as well as to detect the interaction between DNA and proteins (Fig. 4).[54]
 | Fig. 3. (a) The current– voltage curves for a gold nanoparticle wire before (solid line) and after (dotted line) exposure to 1-mM Octadecyl Thiol (ODT)/ethanol solution. (b) The conductance changes of a gold nanoparticle wire prepared by a 5-min deposition through continuous addition of 1-μ M ODT/ethanol solution. (c) The conductance changes of a gold nanoparticle wire prepared using a 10-min deposition after sequentially adding different concentrations of biotin-bovine serum albumin (b-BSA) protein.[47] |
 | Fig. 4. (a) No significant resistance change in gold nanoparticle (14, 27, and 37 nm) wires with different concentrations of single-stranded DNA-binding protein (SSB). (b) The interaction sketch for single-strand DNA and SSB. (c), (d), and (e) Change in Δ R/R values for ssDNAs (600, 1200, and 3200 nucleotides (nt)) upon binding with SSB as a function of SSB concentrations. Gold nanoparticle wires with 37-nm gold nanoparticles were used for all experiments in panels (b), (c), and (d), and ssDNA concentrations were 0.001 nM, 0.01 nM, and 0.1 nM respectively.[54] |
Such high sensitivity results from the exponential dependence of the tunneling resistance on the separation between adjacent nanoparticles. Synthetic colloidal nanoparticles offer great flexibility for performance optimization owing to their varying particle size, capping reagent, and inter-particle distance.
Molecular sensing based on surface plasmon resonance (SPR) of metallic nanoparticles has been widely used. Refractive index changes close to the particle surface caused by mass variation induce changes in SPR-based spectra.[55, 56] Details about immunoassays based on SPR spectroscopy and microscopy have been reviewed, [57, 58] where high-throughput analysis of biomolecular binding kinetics can be studied. Recent works characterized the interactions between gold nanoparticles and proteins and blood cells.[59, 60] Nanoparticle wires that maintain similar molecular sensing capability as that of individual nanoparticles offer additional electrical monitoring capabilities and allow potential multi-modal monitoring. Furthermore, the superior biocompatibility of gold nanoparticles enables potential real-time study of chemical and electrical processes on the membrane of live cells. We anticipate broader biomedical applications of nanoparticle wires for biophysical detection and treatment in the near future.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|
| 43 |
|
| 44 |
|
| 45 |
|
| 46 |
|
| 47 |
|
| 48 |
|
| 49 |
|
| 50 |
|
| 51 |
|
| 52 |
|
| 53 |
|
| 54 |
|
| 55 |
|
| 56 |
|
| 57 |
|
| 58 |
|
| 59 |
|
| 60 |
|


