†Corresponding author. E-mail: gangwang@iphy.ac.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 51322211and 91422303), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB07020100), Beijing Nova Program of China (Grant No. 2011096), and K. C. Wong Education Foundation, Hong Kong, China.
Electrochemical method has been used to insert K/Na into FeSe lattice to prepare alkali-intercalated iron selenides at room temperature. Magnetization measurement reveals that K xFe2Se2 and Na xFe2Se2 are superconductive at 31 K and 46 K, respectively. This is the first successful report of obtaining metal-intercalated FeSe-based high-temperature superconductors using electrochemical method. It provides an effective route to synthesize metal-intercalated layered compounds for new superconductor exploration.
Novel synthetic routes are always highly desired for exploration of new superconductors and other functional materials. This is, in particular, true for new phases that are not obtainable by conventional solid-state reaction routes, while may possess interesting properties. For example, a series of superconductors Ax(NH3)yFe2Se2 (A = Li, Na, Ca, Sr, Ba, Yb, and Eu) have been successfully obtained by using liquid ammonia method, [1– 3] which otherwise cannot be synthesized by the high-temperature route.[4– 12] For Nax(NH3)yFe2Se2, enhanced superconducting transition temperature (Tc) of 46 K was observed.[1] The metal intercalation by other low-temperature methods using pyridine, ethanediamine, and hexamethylenediamine as solvents was then demonstrated to be equivalently effective in inducing superconductivity with similar Tc in Lix(C5H5N)yFe2 − zSe2, [13]Ax(C2H8N2)yFe2 − zSe2 (A = Li, Na), [14, 15] and Lix(C6H16N2)yFe2 − zSe2.[16] Recently, a new superconductor Li0.8Fe0.2OHFeSe with Tc = 43 K was obtained by using a hydrothermal method.[17, 18] This method was also successfully used for obtaining superconducting tetragonal FeS with Tc of 5 K.[19]
Electrochemical synthesis is known to be effective in intercalating Li, Cu, Mg, and Zn into various parent compounds, leading to superconductors respectively.[20– 27] Moreover, electrochemical method can be used to precisely control the content of inserted metals by tuning the transferred electric quantity, which has an advantage over the above-mentioned methods in this context. Considering the structural feature of FeSe, electrochemical method should be possible to intercalate metals in between FeSe layers. Actually, Abe et al.[28] has tried to intercalate Li into FeSe1 − xTex by this method, but failed to induce superconductivity. Chen et al.[29] demonstrated the reentrance of superconductivity with Tc of about 8 K in FeSe-based superconductors by lithium-ion insertion and extraction. Here we successfully intercalate K and Na into FeSe and obtain superconductors KxFe2Se2 and NaxFe2Se2 with respective Tc of 31 K and 46 K by electrochemical method, which is the first successful report on preparation of metal-intercalated FeSe-based high-temperature superconductors by this route. The results demonstrate that the electrochemical synthesis is an effective and facile route for metal-intercalated layered compounds.
We firstly synthesized β -FeSe powders following the method described in Ref. [30]. The electrochemical K intercalation was performed under the constant current mode (10 μ A) and subsequent constant voltage mode (1.04 V) of an electrochemical cell with K metal as the anode and β -FeSe as the cathode. The details of the electrochemical cell are as follows. Saturated KNO3 solution dissolved in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) (EC:DMC= 1:1 by molar ratio) was used as the electrolyte and celgard 2400 as the separator. The working electrode was made of finely ground β -FeSe powders (90 wt.%) and polyvinylidene fluoride (PVDF) (10 wt.%) slurry (total mass is about 3 mg) coated onto an aluminum foil substrate. The electrochemical Na intercalation was performed under a constant current mode (2 μ A) of the electrochemical cell with Na metal as the anode and β -FeSe as the cathode. 1 mol· L− 1 NaClO4 in a mixture of EC and DMC (EC:DMC = 1:1 by molar ratio) was used as the electrolyte and celgard 2400 as the separator. The working electrode was similarly made of finely ground β -FeSe powders (90 wt.%) and PVDF (10 wt.%) slurry (total mass is about 3 mg) coated onto a copper foil substrate. Room-temperature powder x-ray diffraction (PXRD) data were collected using a PANalytical X’ pert Pro diffractometer with Cu Kα radiation. The static magnetic susceptibility was measured using a vibrating sample magnetometer (quantum design).
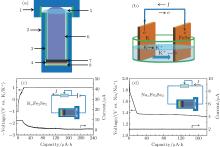
Figures 1(a) and 1(b) show the sketch of electrochemical cell and the schematic of the K intercalation process, respectively. By setting a current loop, the electrons will transfer from K metal to FeSe. Accordingly, K is oxidized to K+ at the surface of K metal. Meanwhile, K+ migrates to and is inserted into FeSe at the working electrode. The concentration of K+ in the electrolyte is maintained at a certain level before K metal is exhausted at the anode. Figures 1(c) and 1(d) show the capacity dependence of the voltage and current measured between working and reference electrodes for these two intercalation processes. As shown in Fig. 1(d), it is a typical discharge curve similar to the discharge reaction in Li-ion batteries. Initially, the potential of the working electrode is positive compared with that of the Na electrode, the current flows from FeSe electrode to Na electrode, which corresponds to Na intercalating to FeSe. The potential quickly decreases down to about 1.4 V at about 20 μ Ah and then a plateau appears, signifying the proceeding of Na intercalation. For the K-intercalation process, an external voltage is applied initially as K cannot be inserted into FeSe through spontaneous discharge process.
Figure 2(a) shows the temperature-dependent magnetic susceptibility of KxFe2Se2. The superconducting transition is evident for both ZFC and FC curves. The Tc is determined to be about 31 K, which is much higher than that of the pristine FeSe, while similar to Tc obtained in solid-phase synthesized K0.8Fe2Se2 at high temperature.[4, 31] The positive magnetic susceptibility seems to be due to magnetic impurities taken into the samples during the intercalation as in Ref. [14]. Whereas the reason for bifurcated ZFC and FC curves is still unknown. The superconductive shielding fraction is estimated to be about 5% at 10 K. Figure 2(b) indicates the temperature-dependent magnetic susceptibility of NaxFe2Se2. The superconducting transition is determined to be 46 K from ZFC and FC curves. It is the highest Tc in metal-intercalated iron selenide superconductors under ambient pressure, which is the same as the Tc observed in ammonia and Na co-intercalated FeSe.[1] It is known that there are two superconducting phases, Kx(NH3)yFe2Se2 (x ≈ 0.3 and 0.6) in the K-intercalated iron selenides, determined mainly by potassium concentration.[32] Moreover, it is found that there is a lower Tc (32 K) phase other than the 46 K phase for Nax(NH3)yFe2Se2 recently.[33] These results indicate that the Tc of AxFe2Se2 (A represents alkali metal) is tunable with varying content of inserted alkali metals. We tried to tune the content of inserted Na by adjusting the duration of discharge process. However, the Tc is unchanged. This may be due to that the intercalation process is inhomogeneous. The alkali metal is only inserted into the surface of FeSe, whereas its further intercalation toward inner parts is hindered by ion diffusion. Precise control of the content of intercalated metals in FeSe is still ongoing with the improvement of the electrochemical process.
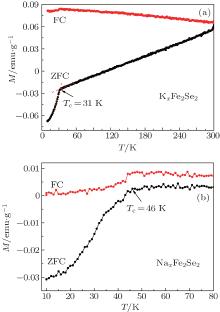 | Fig. 2. Temperature-dependent magnetization of (a) KxFe2Se2 and (b) NaxFe2Se2 in an applied magnetic field of 40 Oe. Arrows indicate the Tc. |
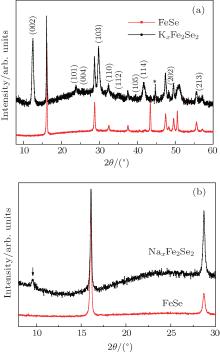
In order to confirm that alkali metal is inserted into FeSe, we performed the PXRD for both samples. In contrast to the PXRD pattern of Li-intercalated FeSe1− xTex previously reported by Abe et al., [28] a new set of diffraction peaks arise for K-intercalated FeSe sample as shown in Fig. 3(a). The new peaks can be well indexed based on the body-centered tetragonal cell (space group I4/mmm) with lattice parameters a = b = 3.898(7) Å , c = 14.07(5) Å . The lattice constants are quite similar to those for solid-phase synthesized K0.8Fe2Se2, [4] indicating that the electrolyte molecule is not intercalated into FeSe, which is different from Kx(NH3)yFe2Se2 obtained by liquid ammonia method.[32] In addition, the coexistence of FeSe indicates that the intercalation is very inhomogeneous, in consistent with the above assumption that K is only inserted on the surface portion of FeSe cathode. For Na-intercalated FeSe sample (Fig. 3(b)), only (002) peak around 9.5° (2θ ) is clearly observed. The lattice constant c given by this peak is about 18.7 Å , which is much larger than 13.6678(4) Å of Na0.65(1)Fe1.93(1)Se2, [34] indicating that not only Na but also electrolyte molecule is intercalated into FeSe. The structural difference between K and Na-intercalated FeSe samples here implies that the size of alkali metals is a key factor in affecting the final structure, reminiscent of the fact that K0.8Fe2Se2 exists, while Na0.8Fe2Se2 does not through solid reaction at high temperature. The synergic effect of co-intercalated electrolyte molecules and Na is believed to stabilize the structure, which is consistent with other Na-intercalated FeSe samples containing both Na and solvent molecules. It should be pointed out that Na0.65(1)Fe1.93(1)Se2 can only exist by carefully extracting NH3 from the structure below around 240 K.[34]
As presented above, electrochemical method can be used to intercalate guest species like alkali metals into host structures like FeSe, which is complementary to the traditional solid-state reaction. Furthermore, comparing to the liquid ammonia and ethanediamine methods, it offers more advantages. For example, it provides the possibility to intercalate more guest species like Mg, [25] Cu, [23] and Zn, [26] which are not dissolvable in those solvent. Furthermore, it should find applications in obtaining more new materials considering its variety by selecting corresponding electrodes and electrolytes. The newly discovered superconducting tetragonal FeS offers a promising platform to explore high-temperature superconductors by the method.[19] The experiments are in progress now.
In conclusion, we have successfully synthesized two alkali-intercalated FeSe materials, KxFe2Se2 and NaxFe2Se2, through electrochemical method. Na can be inserted into FeSe through spontaneous discharge reaction. While an external voltage is initially applied to insert K into FeSe. Structural analysis indicates electrolyte molecules are co-intercalated with Na but not with K into FeSe. Magnetization measurement reveals that the obtained samples are superconducting with Tc of 31 K and 46 K, respectively. It is the first report using this method to get metal-intercalated FeSe high-temperature superconductors. This work provides an effective route to synthesize metal-intercalated layered compounds for new superconductors and other functional materials exploration.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|