† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
§ Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 61204008, 11075176, and 11505211) and the National Key Basic Research Special Foundation of China (Grant No. 2013CB328705).
Role of vacancy-type (N vacancy (VN) and Ga vacancy (VGa)) defects in magnetism of GaMnN is investigated by first-principle calculation. Theoretical results show that both the VN and VGa influence the ferromagnetic state of a system. The VN can induce antiferromagnetic state and the VGa indirectly modify the stability of the ferromagnetic state by depopulating the Mn levels in GaMnN. The transfer of electrons between the vacancy defects and Mn ions results in converting Mn3+ (d4) into Mn2+ (d5). The introduced VN and the ferromagnetism become stronger and then gradually weaker with Mn concentration increasing, as well as the coexistence of Mn3+ (d4) and Mn2+ (d5) are found in GaMnN films grown by metal–organic chemical vapor deposition. The analysis suggests that a big proportion of Mn3+ changing into Mn2+ will reduce the exchange interaction and magnetic correlation of Mn atoms and lead to the reduction of ferromagnetism of material.
Diluted magnetic semiconductors (DMSs) have attracted a lot of attention due to their potential applications in spintronic technology.[1] Among the DMS materials, GaMnN is of particular interest and increasingly studied.[2] However, its potential applications remain far from being fulfilled as various experiments, which leads to controversial conclusions concerning the ferromagnetism in GaMnN.[2–9] Moreover, although Mn is known as forming deep acceptor levels located in the band gap of GaN, the electronic structure and valence states of Mn ions are still controversial,[10–13] and the origin of experimentally observed ferromagnetism is still not fully understood. In our previous experimental studies,[9,14–17] we observed the presence of N vacancy (VN) with the Mn concentration increasing and analyzed the coexistence of Mn3+ and Mn2+ in a GaMnN sample. The understanding of magnetism of GaMnN requires more knowledge of vacancy-type defects and their interactions with the Mn ions. Since current experimental studies cannot resolve the structure and properties of defects to an atomic level, theoretical calculation is a valuable complementary method, because it allows the investigations of the geometry and electronic properties of defects in relative isolation.
Some theoretical studies showed that the Ga vacancy (VGa) gives rise to ferromagnetism in the GaN system.[18,19] However, Mahadevan and Mahalakshmi found that the presence of VGa can destroy the ferromagnetic state in Mn-doped GaN.[20] For N vacancies (VN), few investigations have been performed on the effects of VN on valence states of Mn ions and magnetism in GaMnN. In this paper, first-principle calculations coupled with zero-field-cooled (ZFC) and field-cooled (FC) M–T measurements, are carried out to investigate the changes of the electronic structure and valence states of Mn ions and magnetic properties with the presence of vacancy defects in GaMnN, and to further understand our previous experimental results. In addition, more knowledge of microscopic behavior may be helpful in further progress in the understanding and engineering of the GaMnN.
Our calculation is based on a 32-atom supercell model. The atomic positions and the unit-cell parameters are allowed to relax to the minimum energy configuration to investigate a variety of configurations of Ga15MnN16 (one substitutional Mn atom), Ga15MnN15 (one substitutional Mn atom and one VN), Ga14MnN16 (one substitutional Mn atom and one VGa), Ga14Mn2N15 (two substitutional Mn atoms and one VN), and Ga13Mn2N15 (two substitutional Mn atoms and one VGa). Then, via calculating the system total energy, we choose the stablest structures to study their electronic structures and magnetic properties. Two typical structures are shown in Fig.
All the structure optimizations and density functional theory calculations are performed with the plane-wave pseudopotential method.[21] The Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional under general gradient approximation (GGA) and ultrasoft pseudopotential (Usp) are used to describe the interactions between the valence electrons and the doped ions. The plane-wave cut-off energy is set to be 400 eV, other calculation parameters and calculation steps have been reported in Refs. [22] and [23]. The calculation accuracy has also been evaluated in Refs. [22] and [23].
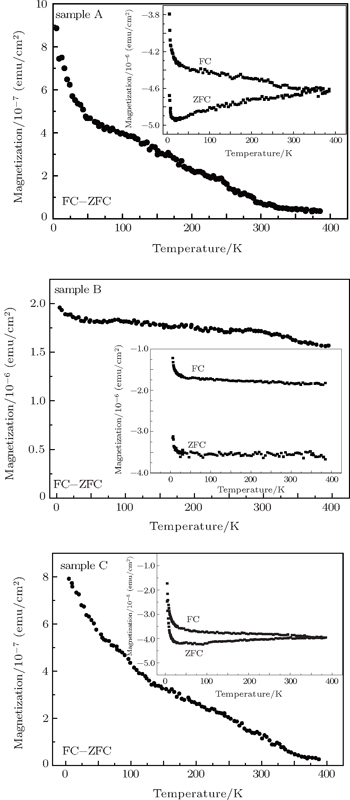
The examined samples here were prepared by metal-organic chemical vapor deposition (MOCVD). The details of the growth conditions have been presented elsewhere.[24] Trimethylgallium (TMGa) and ammonia were used as gallium and nitrogen sources. Bis (monomethycyclopentadienyl) manganese ((MCp)2Mn) was used as a dopant source during the growth of GaMnN films. The Mn concentration of the GaMnN film was controlled by varying the ratio between the molar flow rates of Mn and Ga flowing into the reactor. In this paper, the samples denoted as A, B, and C were fabricated at doping concentration x values of 0.75%, 1.8%, and 2.0%, respectively. Our previous experimental results indicated that no second phase related to Mn was observed for the GaMnN sample in the ω−2θ scan curve and the samples were still long-range lattice ordering and symmetric after GaN had been doped with Mn, which identified that Mn occupied the Ga site in the GaMnN sample.[24,25] The dependences of magnetization on temperature and magnetic-field are investigated using a superconducting quantum interface device magnetometer (SQUID). ZFC and FC M–T curves are obtained at temperatures ranging from 5 K to 400 K in an applied field of 200 Oe (1 Oe = 79.5775 A·m−1).
To better understand the effects of vacancy-type defects on electronic structure and magnetic properties of GaMnN, some of the main details of Mn-doped GaN are recounted here, which has been studied previously.[22] The total and partial density of state of GaMnN are shown in Fig.
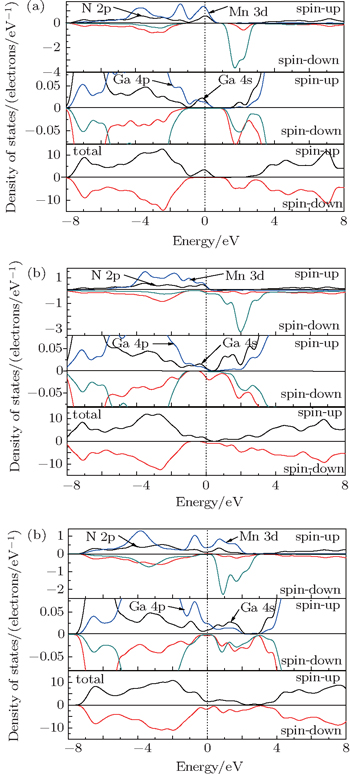 | Fig. 2. Total and partial densities of states for (a) GaMnN, (b) GaMnN:VN and (c) GaMnN:VGa. The upper half for each panel is for spin-up and the other for spin-down. |
| Table 1. Values of magnetic exchange parameter J, and magnetic moment M of Mn and its neighboring Ga and N for the lowest energy structures. . |
Compared with density of states of GaMnN, the partial density of states of Mn 3d electrons has obvious change with the introduction of vacancy defects, and then the total densities of states of these systems have been significantly modified. Figure
Figure
To investigate the charge transfers with the introduction of vacancy-defects in systems, we examine their electron density differences in Fig.
Our previous work[14] and Obloh et al.[29] found that VN was likely to form under the higher growth temperature condition of MOCVD, and that our work identified it as VN-MnGa complex.[14] Moreover, our photoluminescence experiment[17] found that the intensity of yellow band (YL) bands decreased with the increase of Mn concentration in the sample, which was attributed to VGa.[30,31] This result indicates that the VGa concentration will decrease with the increase of Mn concentration in the sample. Another reason is that Mn replaces Ga sites in the GaMnN sample.[32] Our considerations thus should be focused on the VN.
As mentioned above, the presence of VN changes the Mn3+ (d4) configuration in GaMnN. Theoretical study has shown that the Mn–N bond length linearly decreases with the number of the d (Mn) electrons decreasing, which is due to the decreasing of Coulomb repulsion with the nearest N anions. The d (Mn) obtains electrons and Mn3+ (d4) change into Mn2+ (d5), so that the Coulomb repulsion between Mn and the nearest N anions increases. Hence, the calculated equilibrium Mn–N bond length increases from 1.94 to 2.01, the variation of the charge state changes the bond lengths by about 3.6%.[33] In this paper, the calculated values of Mn—N bond length are 1.992 Å and 2.054 Å in GaN:Mn and GaMnN:VN system, respectively, which shows that the bond length increases about 3.1%, which is in agreement with the result in Ref. [33]. The results of the density of state, charge density difference, and the calculated values of the Mn–N bond length, jointly indicate that there appears the Mn2+ (d5) in GaMnN system with the increase of VN, despite Mn replacing Ga in Ga3+N3−. Our previous experiment also found that the Mn3+ (d4) and Mn2+ (d5) coexisted in the sample.[17]
In order to further investigate the VN effects on magnetic properties of the GaMnN thin films, the dependences of magnetization on temperature and magnetic-field are measured by using SQUID. The measurements are performed under ZFC and FC conditions with an applied field of 200 Oe parallel to the film surface.
Figure
In this work, through the first principle calculation, we can infer that the VN or VGa existing in GaMnN influences the ferromagnetic state of the system. The VN can induce an antiferromagnetic state and the VGa indirectly modifies the stability of the ferromagnetic state by depopulating the Mn levels in GaMnN. The additional electrons provided by VN change the d4 (Mn3+) into the filled d5 (Mn2+), the double exchange interaction decreases and antiferromagnetic superexchange becomes dominant. Our previous experiment found that the VN was introduced with Mn concentration increasing and the Mn3+ (d4) and Mn2+ (d5) coexisted in the sample.[14,28] In the present study, the analyses of the ZFC and FC data show that the ferromagnetism of the GaMnN film becomes stronger and then gradually weaker with Mn concentration increasing. Thus, due to the presence of VN, a big proportion of Mn3+ changing into Mn2+ will reduce the exchange interaction and magnetic correlation of Mn atoms, leading to the reduction of ferromagnetism of material. It turns out that the additional VN changes the valence state of Mn ions, which is one of the reasons influencing the magnetic properties of GaMnN. The above analysis suggests that the position of EF and the relative position of Mn levels influence the magnetism of film, while their position closely depends on the optimum growth condition. Perhaps, just the difficulty in controlling the optimum growth conditions in experiment leads to various experimental results in GaMnN. Our work may be helpful for further understanding our previous experimental results and the origin of ferromagnetism in GaN-based diluted magnetic semiconductors.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 |