† Corresponding author. E-mail:
Project supported by the National High Technology Research and Development Program of China (Grant No. 2015AA034201) and the Chinese Universities Scientific Fund (Grant No. 2015LX002).
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is usually sandwiched between indium tin oxide (ITO) and a functional polymer in order to improve the performance of the device. However, because of the strong acidic nature of PEDOT:PSS, the instability of ITO/PEDOT:PSS interface is also observed. The mechanism of degradation of the device remains is unclear and needs to be further studied. In this article, we investigate the in-situ electrochromism of PEDOT:PSS to disclose the cause of the degradation. X-ray photoelectron spectroscopy (XPS) was used to characterize the PEDOT:PSS films, as well as the PEDOT:PSS plus polyethylene glycol (PEG) films with and without indium ions. The electrochromic devices (ECD) based on PEDOT:PSS and PEG with and without indium ions are carried out by in-situ micro-Raman and laser reflective measurement (LRM). For comparison, ECD based on PEDOT:PSS and PEG films with LiCl, KCl, NaCl or InCl3 are also investigated by LRM. The results show that PEDOT:PSS is further reduced when negatively biased, and oxidized when positively biased. This could identify that PEDOT:PSS with indium ions from PEDOT:PSS etching ITO will lose dopants when negatively biased. The LRM shows that the device with indium ions has a stronger effect on the reduction property of PEDOT:PSS-PEG film than the device without indium ions. The contrast of the former device is 44%, that of the latter device is about 3%. The LRM also shows that the contrasts of the device based on PEDOT:PSS+PEG with LiCl, KCl, NaCl, InCl3 are 30%, 27%, 15%, and 18%, respectively.
Since polymer light-emitting devices (PLEDs) were invented by Burronghes et al. at Cambridge University in 1990[1] a great deal of effort has been made to improve the performances of PLEDs, such as lifetime and high luminous efficiency in order to meet commercial requirements. A successful approach to improving the performance of PLED was to insert poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) between the anode (indium tin oxide, ITO) and the light-emitting polymer as a hole transport layer.[2] Similarly, PEDOT:PSS with molecular structures shown in Fig.
The mechanism of the indium effect on the performance of PLEDs is not fully resolved, especially its effects on a biased PLED. Indium ions and/or electrons in PLED will be moved under the action of an electric field when biased, the interaction between indium ions and PEDOT:PSS will occur. However, the thickness of PEDOT:PSS film in PLED is about 50 nm, which is not thick enough to disclose the effect of indium ions. Therefore, an electrochromic device with thicker PEDOT:PSS was used to check the effect of indium ions under the action of an electric field. Here in this paper, indium oxide instead of ITO is used as the substrate etched by the PEDOT:PSS due to its integral role in the etching process.[13] For the first time, we have studied the structural changes of modified PEDOT:PSS film by in-situ micro-Raman and laser reflective measurement and also discuss the effects of indium transfer. For comparison, laser reflective measurements of 25-mM LiCl, 25-mM KCl, 25-mM NaCl, or 25-mM InCl3 modified PEDOT:PSS are also presented.
Indium oxide powder was dissolved with different weight ratios of In2O3 powder (Aldrich product No. 203424) to PEDOT:PSS aqueous solution (Baytron P VP AI 4083 from H.C. Starck) which had been concentrated from 1.3 wt% to 2.76 wt% at 65 °C, and the PEDOT:PSS solution with 0 wt%∼0.3 wt% dissolved In2O3 were successfully prepared. In2O3 powder of more than 0.3 wt% would not be dissolved completely in PEDOT:PSS, and would destroy the stability of PEDOT:PSS. PEG (Aldrich) was first dissolved in DI water and then poured, with the same weight ratio of PEG solid to PEDOT:PSS solid. The relevant PEDOT:PSS films or PEG-PEDOT:PSS films were spin coated on ITO or occasionally on silicon for XPS measurement. The thickness values of films were measured by a surface profiler (Alpha-Step, KLA-Tensor). XPS measurements were performed in an ultrahigh vacuum (UHV) chamber. The UHV system was equipped with a monochromatized Al Kα source and a hemisphere analyzer for XPS. The base pressure of the UHV system was 1.33 × 10−7 Pa and XPS energy resolution was ∼0.6 eV. The binding energy scale was calibrated against the C 1s line centered at 285.0 eV. In-situ micro-Raman measurements were carried out at room temperature by using a Renishaw Raman System 2000. The 514.5-nm line of argon ion laser was used as the exciting light. The spectra were measured in backscattering geometry. The resolution was about 1 cm−1, a 50 × objective was used to focus the laser light on the sample surface into a spot size of 2 μm.
A typical EC device for in-situ micro-Raman measurement is shown in Fig.
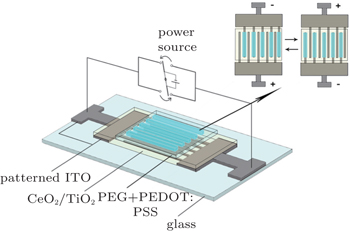 | Fig. 2. Schematic structures of solid state electrochromic devices based on PEG + PEDOT:PSS with and without indium ions fabricated for in-situ micro-Raman measurements. |
Another kind of EC device for laser reflective measurement was made from bottom to top as follows: ITO was used as anode, CeO2–TiO2 film as ion storage layer, PEG-PEDOT:PSS with 0.2 wt% dissolved In2O3 (PEG-PEDOT:PSS-In3+) or without indium ions as the EC layer. For comparison, PEG-PEDOT:PSS samples were mixed with either 25-mM LiCl, 25-mM KCl, 25-mM NaCl or 25-mM InCl3 (all metallic halides from Aldrich). Modified PEDOT:PSS was used as an EC layer while silver served as the cathode. In the device fabrication, the ion storage layer was spin coated to form a film with the thickness in a range from 150 nm to 250 nm on ITO. The EC layer with the thickness in a range from 700 nm to 900 nm was then spin coated on ITO/CeO2–TiO2 and then baked in a vacuum furnace at 80 °C overnight. A silver film was thermally deposited in a vacuum on the top of the EC layer to serve as a counter electrode. The device was covered by a glass plate and sealed with epoxy before removing from the dry box for laser reflective measurement. The bias is defined to be positive if the ITO terminal is connected to the anode. The electrochromic properties were characterized by measuring the reflectivity of light from a red HeNe laser at a 90° deflection angle.
PEDOT:PSS is used as a hole injecting material for the PLED when partially oxidized. It is well established that PEDOT has electrochromic characteristics, for it is transparent when doped and dark blue when undoped. As for PLED, PEDOT is at its oxidation state and displays high conductivity and transparency. The performance of PLED degrades if PEDOT:PSS is reduced when voltage is applied.[18,19] For this reason, it is very necessary to investigate the interaction of indium ion with PEDOT:PSS via an electrochemical method. In order to improve the effect of electrochemistry, PEG is added to help ion transfer.
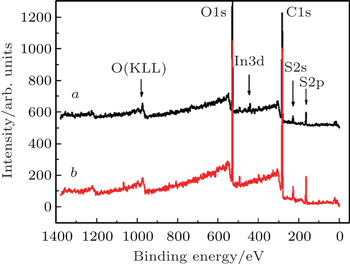 | Fig. 3. XPS survey spectra of (curve a) PEG + PEDOT:PSS with indium ions and (curve b) PEG + PEDOT:PSS without indium ions. |
| Table 1. XPS analyses of PEDOT:PSS, PEDOT:PSS plus PEG and their interactions with indium ions. . |
The solid state electrochromic devices based on PEG-PEDOT:PSS-In3+ and PEG-PEDOT:PSS are measured by an in-situ micro-Raman device. The device structure is shown in Fig.
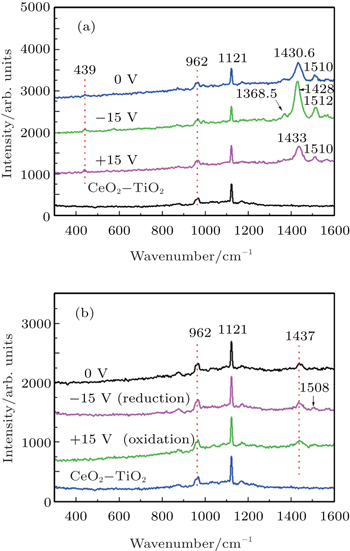 | Fig. 4. in-situ micro-Raman spectra of solid state electrochromic devices based on (a) PEG + PEDOT:PSS+In3+ and (b) PEG + PEDOT:PSS at different applied voltages. |
Compared with Figs.
To further study the effects of indium ions and sodium ions on the properties of PEDOT:PSS laser, the reflective measurements are conducted on solid state electrochromic devices based on PEG-PEDOT:PSS-In3+ and PEG-PEDOT:PSS. The device structures are ITO/PEG-PEDOT:PSS-In3+/Ag and ITO/PEG-PEDOT:PSS/Ag. Similar characterizations of electrochromic devices were reported in previous work.[24] The electrochromism contrasts of the devices each as a function of negatively biased voltage is shown in Fig.
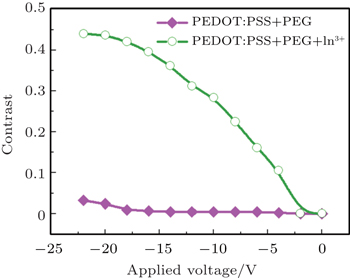 | Fig. 5. Variations of electrochromism contrast with applied voltage, measured at 632.8 nm, for devices with structure ITO/PEG+PEDOT:PSS+In3+/Ag (open circle) and ITO/PEG + PEDOT:PSS/Ag (square). |
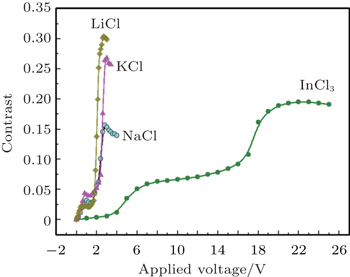
It is also important to investigate the effect of the higher concentration of metallic ions on the electrochromism of PEDOT:PSS. Metallic halides such as LiCl, KCl, NaCl or InCl3 are directly dissolved in PEG-PEDOT:PSS and then electrochromic layers and devices are fabricated. The contrast dependences of applied voltage for the devices based on various metallic halide salts (25-mM LiCl, 25-mM KCl, 25-mM NaCl, 25-mM InCl3 in PEG-PEDOT:PSS solution) are shown in Fig.
It can be seen from Fig.
In this work, PEG-PEDOT:PSS with and without indium ions are prepared in a homogeneous phase. Two kinds of solid state electrochromic devices based on PEG-PEDOT:PSS-In3+ and PEG-PEDOT:PSS are prepared for in-situ micro-Raman and laser reflective measurements. For comparison, laser reflective measurements of LiCl, KCl, NaCl, InCl3 modified PEDOT:PSS are also presented. The results show that the dopant in PEDOT:PSS may be removed when indium ions are introduced. Indium ions have a stronger influence on the structural properties of PEDOT than sodium ions. Indium ions make PEDOT:PSS remove more sodium ions when the device is negatively biased. The laser reflective measurement shows that the contrast of the device based on PEG-PEDOT:PSS-In3+ is 44%. Comparatively, the contrast of the device based on PEG-PEDOT:PSS is only about 3%. The laser reflective measurement also shows that the contrast of the device fluctuates as a function of the metal salt used.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 |