†Corresponding author. E-mail: chunlair@nju.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 91027040 and 21274062).
The interaction of nanoparticles with cell membranes is of great importance because of their potential biomedical applications. In this paper, we investigate the adhesion of stripe-patterned cylinders to a fluid membrane with a full consideration of the Helfrich free energy. Three situations are considered: one striated cylindrical particle, two pure cylindrical particles, and two Janus cylindrical particles. It is found that, with the adhesion of a single sparse striated cylinder, there are a variety of steady-states with energy barriers and the stable state is determined by the pattern of the cylinder. However, when the particle is densely striped, it has no effect on the stable state. By comparing the wrapping degree of two cylindrical particles with that of a single cylindrical particle, we find that two pure cylindrical particles can promote or suppress their interaction with the membrane under different situations. However, two Janus cylindrical particles can only inhibit their interaction with the membrane. Besides, this interaction is related to a first-order transition which is a shallow-to-deep wrapping transition for two pure cylinders while it is a shallow-to-half wrapping transition for two Janus cylinders. Furthermore, the position where the transition happens as a function of adhesion energy is given for fixed membrane tension and the precondition of the transition is presented.
The interaction of nanoparticles with membranes is common in biological systems. A deeply studied example is the way in which viruses pass through their host cell membrane. Typically, they enter the cell by endocytosis and get out of the cell by exocytosis.[1– 4] Therefore, nanoparticles whose sizes are similar to that of viruses have great potential applications in biomedical imaging, and cancer diagnosis and therapy.[5– 9] Acting as drug carriers, they can also transport anticancer drugs to the tumor cells and improve the specific targeting, [10] but the delivery efficiency is still too low to achieve the demand of clinical use. There may also be a potential toxicity because they may disrupt the structure of the membrane.[11, 12] Thus, investigating the interaction between particles and membranes is of great importance to understanding the molecular mechanism of particles passing through cell membranes and designing efficient and safe drug carriers.
With the rapid development of nanotechnology, various nanomaterials can be designed to achieve desirable functionalities. Nanoparticles pass through cell membranes mainly in two ways: endocytosis and diffusion. Their interaction with cells are influenced by the physical and chemical properties of the particles.[13– 22] Micro-sized particles can be engulfed by membranes, [23, 24] while nano-sized particles transport through membranes by diffusion.[25, 26] Dasgupta et al. pointed out that the uptake of ellipsoidal particles is lower than that of spherical particles, which means a higher cell toxicity of tubular viruses than icosahedral viruses.[27]
Particles can be patterned by coating different types of ligands on the surface.[28, 29] It has been shown that the relative arrangements of the ligands influence the interaction between particles and membranes.[30, 31] Using dissipative particle dynamics, Ding et al. found that Janus particles with hydrophobic and hydrophilic ligands can insert into membranes or be engulfed by membranes depending on their initial orientation and properties.[32] Li and coworkers studied the free energy change of four different kinds of ligand-coated nanoparticles piercing through a membrane and found that striated nanoparticle translocated across the membrane with the lowest energy barrier because its rotational degree of freedom was constrained by the anisotropic ligand pattern.[33] More recently, Li et al. have investigated the penetration across a membrane of different patterned nanoparticles. An insertion– rotation penetration mechanism was found for stripy or patchy nanoparticles with a large stripe width or patch size, while a different penetration mechanism with a less constrained rotation was found for stripy or patchy nanoparticles coated with narrow stripes or small patches.[34] These researches are performed by computer simulations and they have made significant progresses. But there are time and length limitations for computer simulations. Studying the interaction between patterned particles and membranes in theory will help us better understand this problem generally.
Most theoretical studies on the particle– membrane interactions are based on the Helfrich model which treats the membrane as a soft bendable sheet and includes two parameters: the bending rigidity and the surface tension. Within the framework of the Helfrich free energy, Deserno et al. studied the adhesion of a single spherical particle to a flat fluid membrane and observed a second-order adsorption transition and a first-order partial-to-complete wrapping transition.[35] Boulbitch found a binding transition by analyzing the free energy of the membrane adhered by a cylinder.[36] Niu and coworkers studied the adhesion of two rigid cylinders to a membrane tube and found a second-order and a first-order transition.[37] Mkrtchyan et al. showed that a shallow-to-deep wrapping transition exists by studying the free energy of two cylinders adhering to the same side of a fluid membrane and concluded that a membrane-mediated repulsion exists between the two cylinders.[38]
In this paper, we focus on the adhesion of striated cylindrical particles to a fluid membrane without spontaneous curvature. In Section 2, we will present the theoretical model and derive the free energy of the entire system based on a full consideration of the Helfrich model. Then, the effect of patterns will be studied by analyzing the free energy of the system in Subsection 3.1. In Subsection 3.2, the interaction between two cylinders is given and a first-order transition will be found from a perspective of the wrapping angle in steady state.
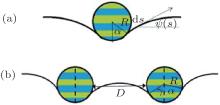
A cross section sketch of a single striped cylinder and two parallel striped cylinders adhering to a membrane is shown in Fig. 1, where the adhesion angles α and γ are well defined. The membrane is represented by a curve and its shape can be described by the tangent angle ψ (s) completely. Different stripes can adhere to the membrane with different adhesion energies and they divide the cross diameter of the cylinders into N parts equally. A simple model of a cross-changing adhesion energy is studied here. As illustrated in Fig. 1, the adhesion energy per unit area of the blue region is − ω 1, while the green region is − ω 2. We assume that the length of the cylinders L is much larger than the radius of section R and ignore the variation of the membrane shape in the direction vertical to the cross section, so that the system can be studied by a two-dimensional model. But we should notice that for real application, it is also of great importance to investigate the effect of aspect ratio of cylinders on the cellular uptake. However, this is beyond the frame of the present work, and we suggest the reader to refer to the recent simulation work by Yue et al. for more details about the related problem.[39]
We assume that the membrane is planar before the cylinder adheres to it. This is reasonable because the size of the membrane can be much bigger than the particles. Adsorption of the cylinder changes the shape of the membrane. The energy difference of the membrane is the integral over the local bending and tension contributions and can be described on the basis of the Helfrich model[40– 42]
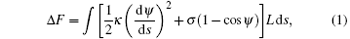
where κ is the bending rigidity and σ is the surface tension.
For the adhesion of a single striped cylindrical particle to a membrane (as illustrated in Fig. 1(a)), the adhesion energy of the system can be written as
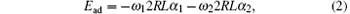
where α 1 and α 2 are the wrapping angles occupied by the blue and green regions respectively in the wrapping area and we have
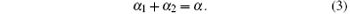
It is convenient to express energies in the units of κ and lengths in the units of the cylinder radius R. So we introduce the following dimensionless quantities
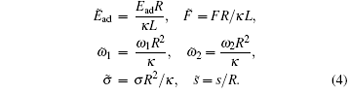
Then the rescaled adhesion energy of the striped cylinder can be expressed as
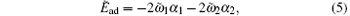
and the rescaled energy difference of the membrane is:
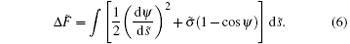
The membrane is divided into two parts (i.e., adhering portion and non-adhering portion) based on whether or not it is in contact with the cylinder. It is clear that
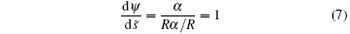
in the contact area. Then equation (6) can be integrated and turns to
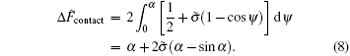
The total energy of the system that a single striped cylinder adheres to a membrane is:
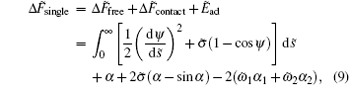
where Δ F̃ free is the rescaled energy difference of the free membrane. The stable structure of the entire system can be determined by minimizing this total energy with respect to the shape function ψ s.
The integral term in Eq. (9) corresponds to a Lagrangian:
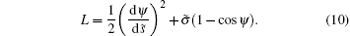
Introducing the related Hamiltonian and using the Euler– Lagrange equation, we have:
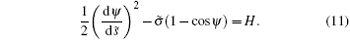
Here H is a constant because L is independent of the arc-length s. This equation can convert the integration over arc-length s to the integration over tangent angle ψ and forms the basis for the deductions below.
The membrane becomes an asymptotical flatness when it is far away from the contact area. This means that
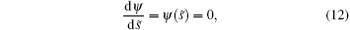
when s̃ = ∞ .[38] So H = 0 in Eq. (11) for the free membrane and
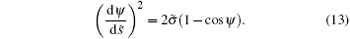
By substituting it to Eq. (6), we get the rescaled energy difference of the non-adhering portion
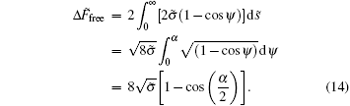
The total energy in Eq. (9) turns to
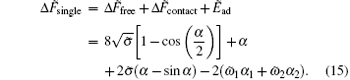
It can be also used to describe the adhesion of a pure cylinder to a membrane when we set the adhesion energy of unit area as ω = ω 1 = ω 2.
The maximum adhesion angle of a pure cylinder can be calculated by analyzing the distance between the left and right of the wrapping membrane
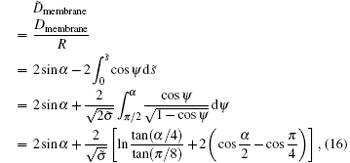
where ψ (0) = α represents the contact point of the free membrane with the cylinder and ψ (s̃ ) = π /2 represents the closest point of the two membrane sides.[38]
For the adhesion of two parallel striped cylinders to a membrane, where the surface-to-surface distance between the two cylinders is D (as illustrated in Fig. 1(b)). The adhesion of the left side of the left cylinder is same to that of the right side of the right cylinder in this symmetrical system. Two vertical diameters of the cylinders divide the system into two portions: (i) the outer part including the left side of the left cylindrical diameter and the right side of the right cylindrical diameter, where the adhesion angle is α , (ii) the middle part between the two diameters, where the adhesion angle is γ . Then the dimensionless energy of the system can be expressed as

It is clear that the adhesion of the outer part of the two cylinders is identical with that of a single cylinder

So we mainly focus on the middle part between the two cylinders. The shape of the membrane in the middle part also conforms Eq. (11). The symmetric point of the middle part satisfies ψ = 0. If we assume that the curvature of this point is ζ , then we get the constantH = ζ 2/2 in Eq. (11) and
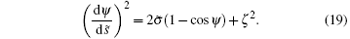
Similarly, we can get the the rescaled energy difference of the middle part of the two striped cylinders
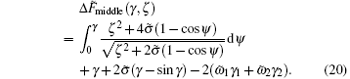
Here, γ 1 and γ 2 are the wrapping angle occupied by the blue and green regions, respectively, in the middle wrapping area. When we set ω 1 = ω 2 = ω , we can study the adhesion of two pure cylinders to a membrane.
On the basis of the shape function Eq. (11), the rescaled surface-to-surface distance between two cylinders can be expressed as
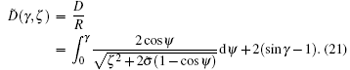
The steady state of the middle part can be got by minimizing the free energy in Eq. (20) with respect to γ and ζ together with the constraint (21).
As a final note, we assume that the cylinder does not turn over during the process of adhesion. It is challenging to consider within the Helfrich model and needs further studying.
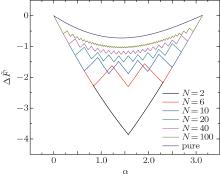
Firstly, we study the influence of the stripes in the adhering of a single cylinder to a membrane. The dimensionless energy difference as a function of wrapping angle for various numbers of stripes is shown in Fig. 2. From bottom to top, the number of stripes is N = 2, 6, 10, 20, 40, 100, and N = 1 (which represents a pure cylinder without stripes), respectively. The rescaled membrane tension σ ̃ = 0.1 is fixed. The rescaled adhesion energies per unit area of the patterned cylinder are ῶ 1 = 2 and ῶ 2 = 0, while that of the pure cylinder is 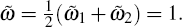
When N = 2, the energy curve has a minimum at α = π /2 which means that a Janus cylinder will be half-wrapped by the membrane. When the cylinder is sparsely patterned, take N = 6, 10, 20 for example, the energy curve is serrated and there exist a variety of steady-states with huge energy barriers. The favorable adhesion angle is small and is dependent on the patterns of the particles. When the number of stripes is big enough, for example N = 100, the energy curve of the dense-striped cylinder is similar to that of the pure cylinder. Their wrapping angles of the steady-states are the same, which means that dense-striped patterns have barely no effect on the wrapping.
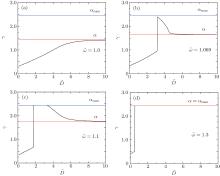
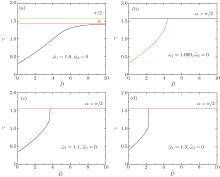
In this section, we discuss the interaction of two cylindrical particles adhering to a membrane by comparing the wrapping angles of the system with that of a single cylindrical particle. In Figs. 3(a)– 3(d), the wrapping angle γ as a function of their surface-to-surface distance D̃ is displayed, where the rescaled membrane tension σ ̃ = 0.1 is fixed and the rescaled contact energy per unit area ῶ is 1.0, 1.069, 1.1, and 1.3 respectively. α is the wrapping angle of a single particle under the same situation and α max is the maximum wrapping angle in which the particle will be completely wrapped.
When ῶ is small, the intercylinder wrapping angle increases while increasing the surface-to-surface distance and reaches to α at last (as illustrated in Fig. 3(a)). This proves that the interaction between the two cylinders disappears when the distance of them is long enough. At the same time, the wrapping angle in the middle portion of two cylinders is always smaller than that of a single cylinder, which means that the two cylinders inhibit their interaction with the membrane.
For larger adhesion energy (as illustrated in Figs. 3(b) and 3(c)), the wrapping angle γ firstly increases continuously as the distance increases. Then at certain point, the wrapping angle γ jumps from a small value to a big value abruptly, which infers a first-order transition from shallow to deep or even complete wrapping. The transition has already been observed by Chen et al.[38] by analyzing the free energy of the middle part and our results are in accordance with their findings. Before the transition point, the intercylinder wrapping angle γ is smaller than the wrapping angle of a single cylinder α , which means that the two cylinders inhibit their interaction with the membrane when the distance is short. Meanwhile, after the transition point, the angle γ becomes bigger than α , which means that the interaction between the two cylinders is promotive. When we further increase the distance, the angle decreases and finally reaches to α asymptotically, which reflects that the distance between the cylinders is too far to influence their interaction.
When the adhesion energy is large enough so that a single cylinder can be completely wrapped by the membrane, the intercylinder wrapping angle γ jumps from a small value to α max as the distance D̃ increases and remains unchanged after the transition point (as illustrated in Fig. 3(d)). This corresponds to a transition from shallow to complete wrapping. The two cylinders inhibit their interaction with the membrane before the transition point and have no interaction after the transition point.
These results demonstrate that the interaction between the two cylindrical colloids and the membrane depends on the adhesion energy and the distance of the two cylinders. The promotive interaction can be observed only if the adhesion energy is big enough so that a first-order transition exists. An inhibitive interaction between the two cylinders changes to a promotive one when the distance D̃ moves across the transition point.
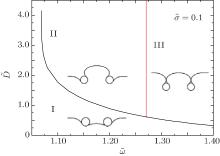
To further understand the first-order transition from shallow wrapping to deep wrapping, we change the adhesion energy and distance systematically, and show the phase diagram in Fig. 4. The rescaled membrane tension σ ̃ = 0.1 is fixed. The red line corresponds to the adhesion energy per unit area in which a single cylinder can be completely wrapped by the membrane. The bottom-left region of the black line (I) corresponds to the shallow wrapping where the transition has not happened. At this time, the intercylinder wrapping angle is smaller than that of a single cylinder so that the two cylinders inhibit their interaction with the membrane. While the top regions (II and III) correspond to the deep and even complete wrapping where the transition has happened. The intercylinder wrapping angle is bigger than that of a single cylinder, so that the two cylinders promote their interaction with the membrane in region II. But the intercylinder wrapping angle is equal to that of a single cylinder where they both equal α max in region III. So that there is no effect for wrapping in this region.
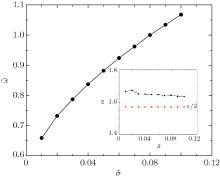
We also find that the transition can happen only if the adhesion energy per unit area is large enough for a given value of membrane tension σ ̃ . As shown in Fig. 4, the transition starts approximately at ῶ = 1.07. Obviously, this adhesion energy where transition starts varies for different membrane tensions. The line in Fig. 5 reflects the transition starting points for different tensions quantitatively. The area below the line corresponds to systems that transition cannot happen no matter how big the surface-to-surface distance is, where the two cylinders can only inhibit their adhesion with the membrane. While the area above the line corresponds to systems that transition can happen when the surface-to-surface distance is appropriate. The two cylinders inhibit their adhesion with membranes before the transition happened while improve their adhesion after the transition happened. By further studying the adhesion angle of a single cylinder in the values of this line (see the inset in Fig. 5), we find that the wrapping angles of a single cylinder are all near to and a little bigger than π /2. This means that a first-order transition of shallow to deep wrapping between two cylinders can happen only if a single cylinder can be at least half-wrapped by the membrane under the same conditions.
In this section, we discuss the interaction of two Janus cylinders whose top half adhesion energy per unit area with the membrane is zero so that the maximum adhesion angle is π /2. The wrapping angle γ as a function of D̃ is displayed in Figs. 6(a)– 6(d), where the rescaled membrane tension σ ̃ = 0.1 is fixed. To compare with the results of two pure cylinders discussed before, we take the rescaled adhesion energy of the lower half part as 1.0, 1.069, 1.1, and 1.3, respectively, and zero in the upper half. Similarly, α is the wrapping angle of a single Janus particle under the same situation.
When the adhesion energy is small (see Fig. 6(a)), the wrapping angle increases as the surface-to-surface distance between the Janus cylinders increases and at last reaches to α . The two Janus cylinders inhibit their interaction with the membrane, which is same to that of the two pure cylinders illustrated in Fig. 3(a).
When the adhesion energy is big enough so that the wrapping angle of a single Janus cylinder can reach to π /2, the intercylinder wrapping angle reaches to π /2 finally. Interestingly, we observe two situations, i.e., the wrapping angle γ increases to π /2 smoothly when the adhesion energy is not so big (see Fig. 6(b)), and the wrapping angle γ jumps from a little value to π /2 abruptly when the adhesion energy is much bigger (see Figs. 6(c) and 6(d)).
A transition of shallow-to-half wrapping exists during increasing the surface-to-surface distance of the two Janus cylinders. Unlike the results of two pure cylinders discussed previously, the adhesion angle of the two Janus cylinders cannot be bigger than that of a single one no matter whether the transition happens. This means that the two Janus cylinders can only suppress their interaction with the membrane.
The intercylinder free energy difference of two pure cylinders is shown in Fig. 7(a), which is the same as that in Ref. [38]. Chen and coworkers pointed out that two branches of the free energy exist for large adhesion energy, corresponding to shallow and deep wrapping, respectively.[38] This reflects a competition between the adhesion energy, which drives the adhesion, and the membrane free energy, which opposes the adhesion. For the free energy difference of two Janus cylinders in Fig. 7(b), two branches exist similarly, which corresponds to a transition of shallow-to-half wrapping.
In the present study, with a full consideration of the Helfrich free energy, we firstly investigated the adhesion of a stripe-patterned cylinder to a fluid membrane. By comparing the energy difference of the system with various number of stripes, we have concluded that the patterns of the cylinder influence the steady state when the stripes are sparse, while they have no effect when the cylinder is dense-striped.
Secondly, a first-order phase transition from shallow wrapping to deep or complete wrapping in the case of two pure cylinders has been observed. The distance between two cylinders at the transition point decreases as the adhesion energy increases when the membrane tension is fixed. The adhesion energy of transition starting point for different membrane tensions has also been calculated and we have found that the transition can happen only if a single cylinder can be at least half-wrapped by the membrane under the same conditions. Compared with the adhesion of a single cylinder, two pure cylinders suppress their adhesion with the membrane before the transition point and may promote the adhesion after the transition happened. For two Janus cylinders, a first-order phase transition of shallow to half wrapping has been found and they can only inhibit their adhesion with the membrane.
These results will be helpful to understand the process of drug delivery and will provide theoretical guidance for designing appropriate drug carriers.
We are grateful to the high performance computing center of Nanjing University for doing the numerical calculations in this paper on its IBM blade cluster system.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|