† Corresponding author. E-mail:
Project supported by President’s Fund of CUHKSZ, Longgang Key Laboratory of Applied Spintronics, at The Chinese University of Hong Kong, the National Natural Science Foundation of China (Grant Nos. 11974298 and 61961136006), the Shenzhen Fundamental Research Fund, China (Grant No. JCYJ20170410171958839), and Shenzhen Peacock Group Plan, China (Grant No. KQTD20180413181702403).
We report a p24 (HIV disease biomarker) detection assay using an MgO-based magnetic tunnel junction (MTJ) sensor and 20-nm magnetic nanoparticles. The MTJ array sensor with sensing area of 890 × 890 μm2 possessing a sensitivity of 1.39 %/Oe was used to detect p24 antigens. It is demonstrated that the p24 antigens could be detected at a concentration of 0.01 μg/ml. The development of bio-detection systems based on magnetic tunnel junction sensors with high-sensitivity will greatly benefit the early diagnosis of HIV.
Early disease diagnoses require detection and quantification of disease biomarkers in serum, urine, or other bloody fluids. The traditional fluorescent immunoassay method suffers from the fluorescent background of biological components, fluorescence quenching and photobleaching of the fluorescent probes which are photo-sensitive.[1,2] Benefited from the recent advancement of nanotechnology and thin-film technology, the magnetic immunoassay (MIA) utilizing magnetic nanoparticles and magnetoresistive sensors has attracted increasing attention in the biomedical area, due to its high sensitivity, low power consumption, low cost, and feasibility to be miniaturized. Since most biological systems do not exhibit significant magnetic background and the magnetic properties of magnetic nanoparticles are not subject to photo-bleaching, the magnetic immunoassay can exhibit stable and reliable performance with high signal-to-noise ratio.[3]
Magnetic tunnel junction (MTJ) sensors, one advanced kind of MR sensors, are recognized as an ideal sensor element for the biodetection due to their low cost, high sensitivity, and lab-on-chip compatibility. Compared with giant magnetoresistance (GMR) sensors, MTJ sensors offer even higher MR ratio (e.g., 604% at room temperature) and therefore potentially higher sensitivity at low magnetic field (especially for the magnetic field < 1 Oe).[4,5] Besides the magnetic field detector, the magnetic iron oxide nanoparticles (IONPs) have been a popular choice as bio-labels due to their physical and chemical stability, low toxicity, environmental safety, and being inexpensive to produce.[6–8] The biodetection configuration utilizing IONPs and MTJ sensors has successfully been applied for bimolecular recognition and DNA detection in previous studies.[9–11] Recently, a dynamic range of 4 orders of magnitude and a limit of detection of 1 pM and 0.1 pM respectively for 15- and 18-cycle amplified synthetic GAPDH PCR products were realized in GMR sensor array platform with magnetic nanoparticles.[12] These developments show the reliability of magnetoresistive immunoassay techniques in biological detection area. In this work, we demonstrate feasibility of magnetic biodetection for p24 antigens using carboxyl-group functionalized magnetic IONPs with MTJ array sensors.
We take p24 antigens here as biotarget due to the fact that p24 antigen is the biomarker of HIV disease. Acquired immunodeficiency syndrome (AIDS) is the end-stage disease caused by human immunodeficiency virus (HIV) infection.[13] Early diagnoses of HIV and thus early treatment of patients have been confirmed to bring dramatic survival benefits, while unfortunately the early detection of HIV remains problematic. In recent years, it is evidenced that the individuals newly infected with HIV have transmitted the virus prior to knowing their HIV status. In the newly infected individuals, HIV antigen p24 is usually present in their serum or plasma 7–10 days before the HIV antibody. The detectable amount of p24 antigens in serum/plasma is in the pg/ml range.[14] Thus, the development of a rapid quantitative bio-detection system with high-sensitivity to detect the p24 antigen will greatly benefit the early diagnosis of HIV.
For the magnetic detection of p24, both the IONPs and MTJ sensors need to be surface biologically functionalized. The magnetite iron oxide nanoparticles synthesized with carboxyl groups were used for the immobilization of the detecting antibodies via covalent bonding.[15] Anti-p24 antibodies were purchased from Abcam. The iron oxide nanoparticles (IONPs) were characterized under TEM observation and their magnetic property measurement was carried out at room temperature using a vibrating sample magnetometer (Lakeshore, VSM 7400). The magnetization was measured over a range of applied field from around – 10000 to 10000 Oe.
The IONPs with carboxyl groups were conjugated with antibodies via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) coupling chemistry.[16,17] Then the nanoparticles are incubated with detecting antibody and resuspended in storage buffer (PBS, phosphate buffered saline). The detailed procedure to carry out the biofunctionalization of IONPs with detecting antibodies is illustrated in Fig.
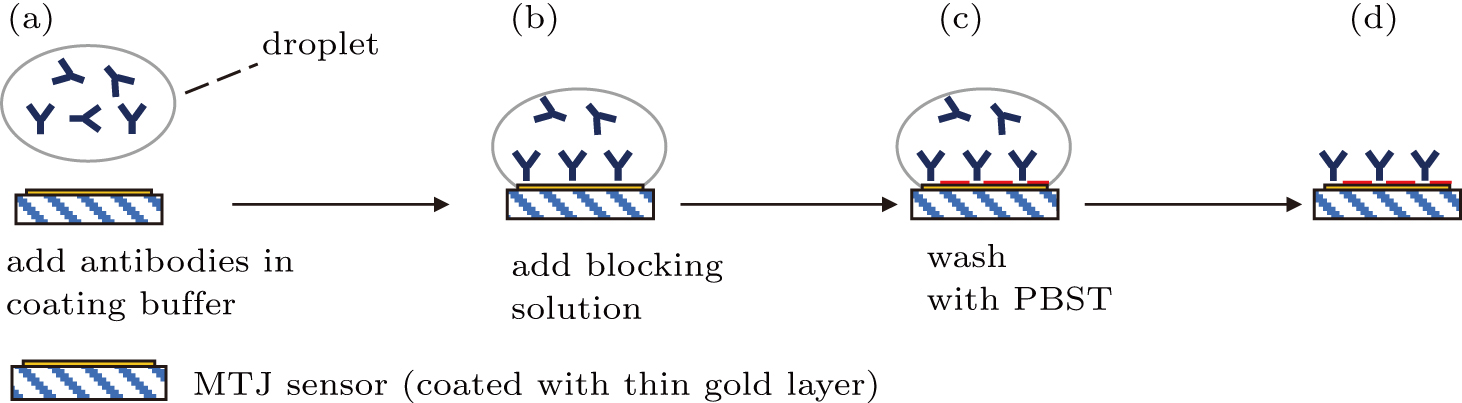
An MR sensor was utilized in this platform to detect the magnetic signal of magnetic biolabels, including single MTJ sensor and MTJ array sensor (both obtained from Micro Magnetics), of which the surfaces were immobilized with capturing antibodies. An MTJ array sensor was bio-functionalized with anti-p24 antibodies. A 200-nm-thick gold layer was deposited on the top of MTJ sensing area, serving as bioactive area. The characterizations of a single MTJ sensor and the MTJ array sensor were carried out, respectively, through four-point probe measurement and two-point probe measurement. The detecting antibodies were immobilized on the MTJ sensing area through the procedures as shown in Fig.
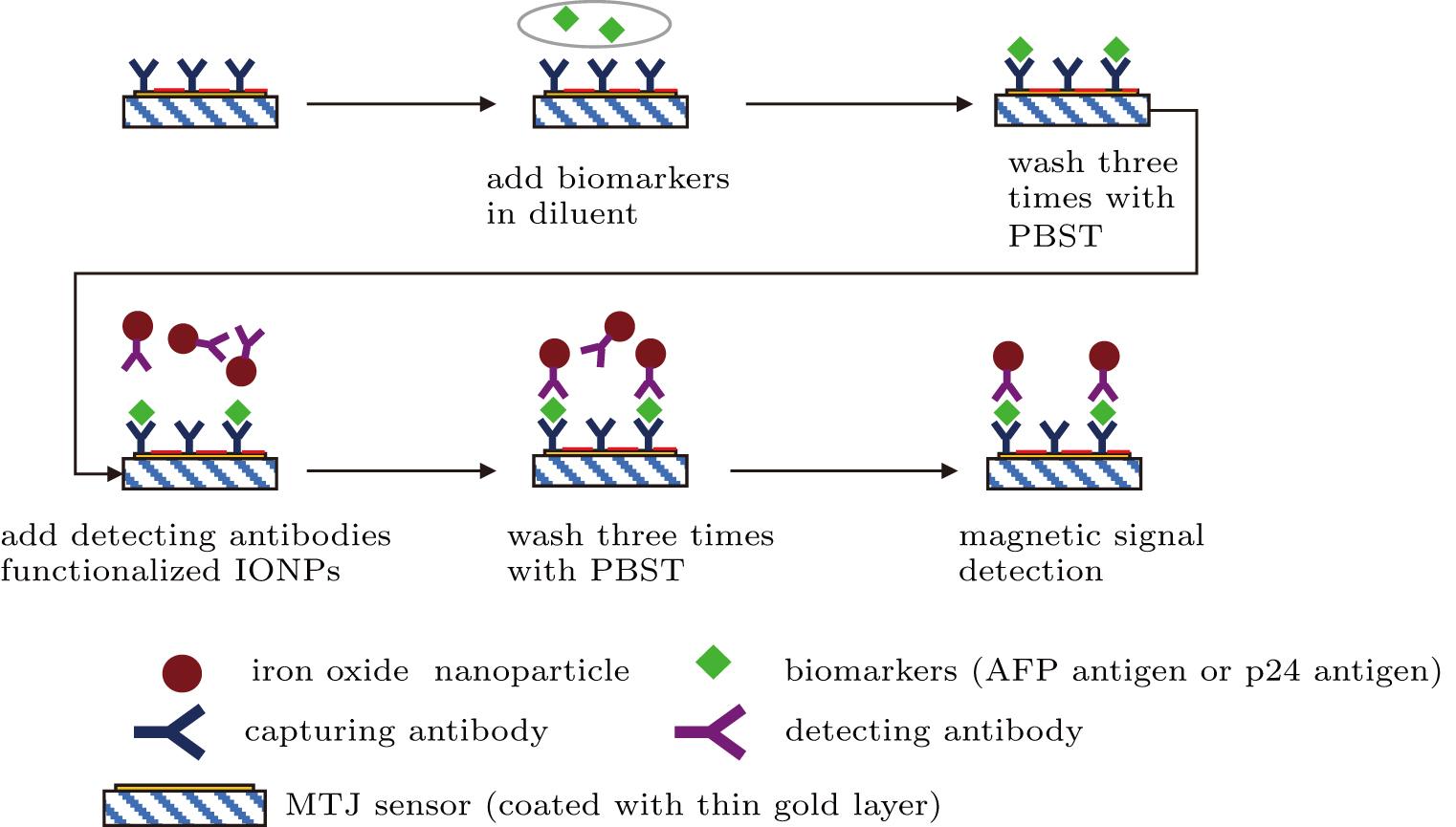
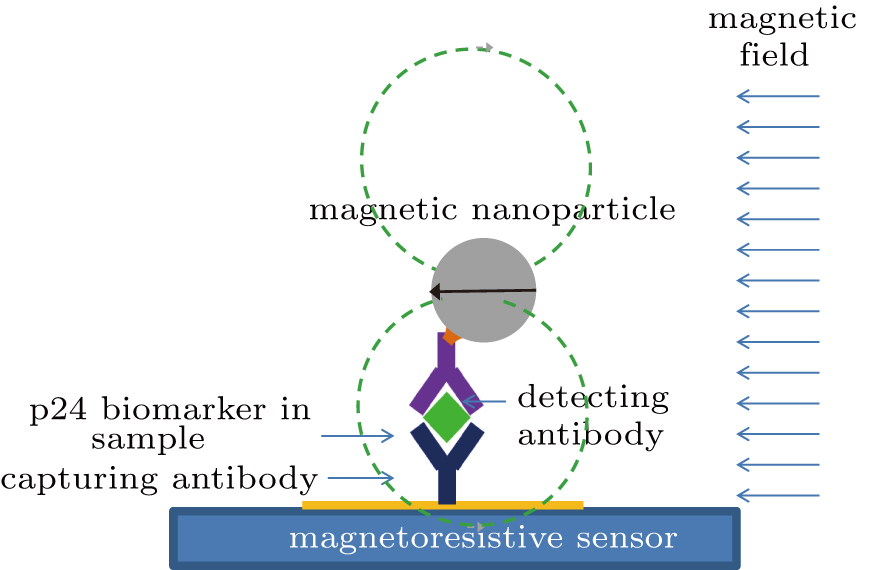
After the biofunctionalization of the IONPs with detecting antibodies and the MTJ sensor with capturing antibodies on its sensing area, the detection of target antigen biomolecules was carried out through a sandwich immunoassay configuration as illustrated in Fig.
Then the sensor surface was washed with PBST after the full binding of antigens with capturing antibodies immobilized onto the MTJ sensor surface. Subsequently, the detecting antibody functionalized IONPs were introduced, and bound to the target antigens at the different epitopes from the capturing antibodies. After removing the excess IONPs by flushing the sensor surface with PBST, a hard-axis field was applied for polarizing and magnetizing the IONP. The stray magnetic field of IONPs was detected by the MTJ sensor, and the signal acquisition was performed on the MTJ array sensor through two-point probe measurement.
To acquire high sensitivity in magnetic biodetection, the IONPs as bio-labels are required to be superparamagnetic with low remanence ratio and high saturation magnetization. They need to generate strong enough magnetic signals to be detected by magnetic sensor, while the particle agglomeration can be avoided when there is no applied magnetic field. The IONPs with carboxyl groups were synthesized characterized as described previously.[20] They exhibited superparamagnetic behavior with a relatively higher saturation magnetization (Ms) of around 50 emu/g when displaying a spherical morphology with size of 20 nm.
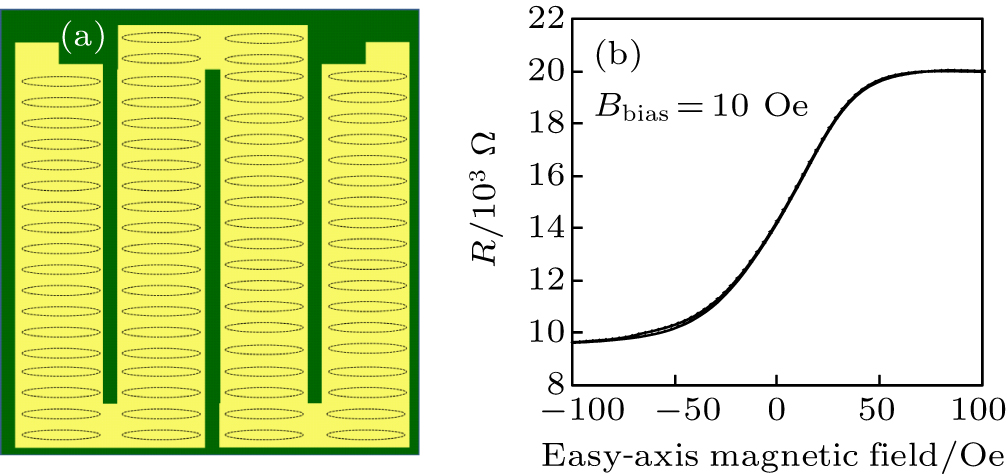
The MTJ array sensor (purchased from Micro Magnetics) consists of 76 junctions, and every junction stack was prepared by magnetron sputtering on thermally oxidized silicon wafers with a base pressure of 2 × 10−10 Torr. The thin-film stack structure was (in units of nm): substrate/Ta (5)/Ru (30)/Ta (5)/CoFe (2)/IrMn (15)/CoFe (2)/Ru (0.8)/CoFeB (3)/MgO (1.9)/CoFeB (3)/Ta (5)/Ru (10). The MTJ array sensor was schematically drawn in Fig.
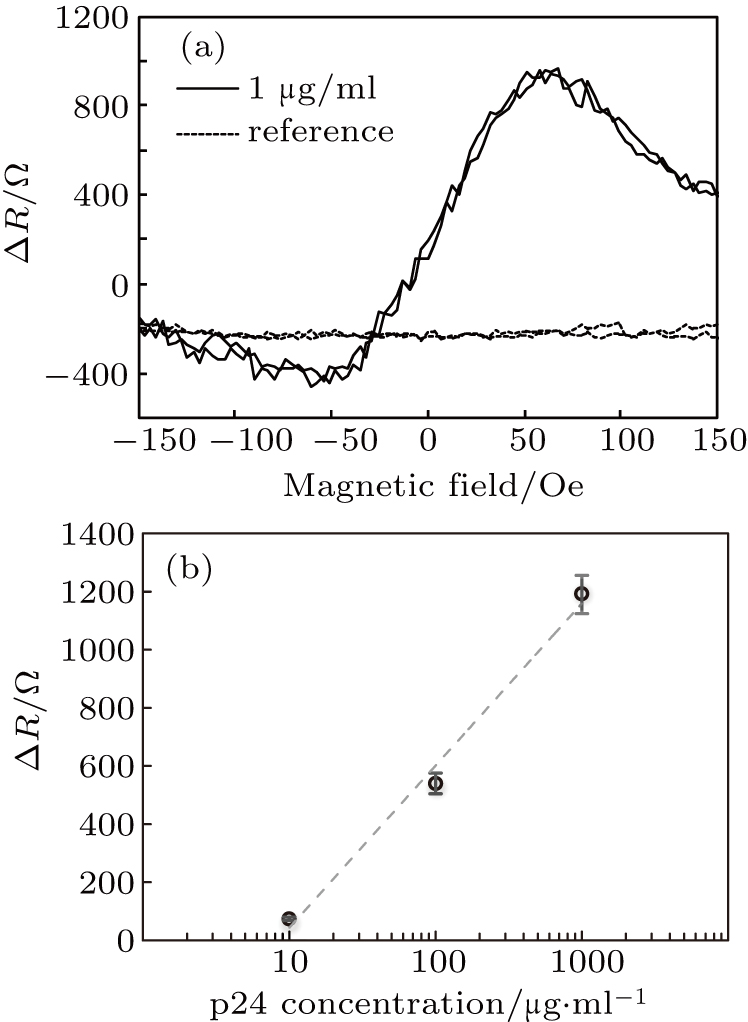
The detection of target p24 biomolecules was realized through the two-point probe measuring configuration with the MTJ array sensor and biofunctionalized IONPs. As illustrated in Fig.
For the p24 with concentration of 1 μg/ml, the resistance variations ΔR of the sensor against easy-axis field changed is shown in Fig.
Thus, here we demonstrated the feasibility of sensitive detection of p24 antigens using carboxyl-group functionalized IONPs with the MTJ array sensor. This logarithm relation is consistent with the previous works reported for biosensing using magnetic nanoparticles and MR sensors.[23]
In summary, we have demonstrated the feasibility of HIV disease immunoassay with carboxyl-group functionalized IONPs and an MTJ sensors by a sandwich-assay configuration. The MTJ sensors and IONPs were biologically treated with capturing antibodies and detecting antibodies before the magnetic biodetection experiments. The MTJ array sensor with a sensing area of 890 × 890 μm2 (almost the whole surface area of sensor) possessing a sensitivity of 1.39%/Oe was used to detect p24 antigens, HIV disease biomarker. It is demonstrated that the p24 antigens could be detected at a concentration of 0.01 μg/ml (10 ng/ml).
Furthermore, the magnetic biodetection of p24 antigens for health care is firstly realized here based on the configuration of MTJ sensors and carboxyl-group functionalized IONPs. The lower limit of p24 tested in our experiment is 0.01 μg/ml. However, the lower limit of detection in the regular HIV-1 p24 antigen test is reported to be 10 pg/ml, a concentration approximately reaches between three and four weeks after infection.[24] The commercially available fluorescent assay kits to detect p24 antigen with limits of detection is reported to be from 11.9 pg/ml to 33.5 pg/ml.[25] It has been demonstrated by researchers that the sensitivity of biodetection based on GMR sensor is superior to fluorescent immunoassay, and MTJ sensors offer higher sensitivity at low magnetic field than GMR sensors.[22,26] Our future work is to optimize this detection configuration with MTJ sensors and to improve its detection sensitivity for p24 antigens.
In addition, the multiplex sensing and miniaturization are also a very valuable future work for our magnetic biodetection configuration. The multiplex sensing of different bioanalytes can be realized by pre-mobilizing different capturing molecules on different working areas on an MR sensor array.[27] The compatibility of MR sensors with the standard CMOS fabrication technology enables great promise for our magnetic biodetection platform to be miniaturized in lab-on-chip applications for point of care with high sensitivity and low cost.[28]
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] |