† Corresponding author. E-mail:
Project supported by the Project of Ph. D. Special Research of Sichuan University of Arts and Science, China (Grant No. 2019BS006Z) and the Fund from the Chinese Academy of Sciences (Grant Nos. KJCX2-SW-N03 and KJCX2-SW-N20).
The equation of state (EOS) of Cr3C2 at high pressure is studied by the synchrotron radiation x-ray diffraction (XRD) in a diamond anvil cell (DAC) at ambient temperature, and density functional theory (DFT). The XRD analysis shows that the orthorhombic structure is maintained to a maximum pressure of 44.5 GPa. The XRD data show that the bulk modulus is K0 = 292 (18) GPa with 
Crystal Cr3C2 is a kind of high melting point material with good wear resistance, corrosion resistance, and oxidation resistance in high temperature environment. It can be widely used in aircraft engines and petrochemical mechanical devices, and can greatly improve the service life of machinery.[1–3] So far, Cr3C2 has aroused the great interest of researchers.[4–10] Kuriyama et al. investigated the electrical conductivity of the Cr3C2 at different sintering temperatures.[4] Ma et al. obtained the Cr3C2 with a hardness Hv = 18 GPa.[5] Hirota et al. derived a hardness of Hv = 18.9 GPa for Cr3C2.[6] Min et al. calculated the structure, electronic properties, etc. of Cr3C2 by using density functional theory.[7] Li et al. calculated a hardness of 20.9 GPa of Cr3C2 by using the first principles calculations.[8] Jiang investigated the structure, elastic and electronic properties of Cr3C2 by using first principles calculations.[9] Jiang et al. obtained a hardness of 20.3 GPa for Cr3C2.[10]
Pressure can reduce the atomic distance, change the electronic shell state and crystal cell structure, and then change its structure, physical and chemical properties, such as high-pressure structural phase transition, high-pressure strength, high-pressure texture.[11] High pressure science is an interdisciplinary science, which has been integrated with physics, material science, geoscience, chemistry, and other disciplines, and thus greatly promoting its application and development and its relevant fields as well, and it is an important field of basic research and applied science research. The physical and chemical properties of Cr3C2 under high pressure are closely related to its crystal composition. Therefore, a thorough understanding of the crystal composition of Cr3C2 is helpful in improving the scientific use of Cr3C2.
Although the Cr3C2 has been studied,[4–10] there is no direct experimental measurement nor theoretical calculation for its high-pressure EOS. Therefore, the equations of state (EOSs) of Cr3C2 under the pressures up to 44.5 GPa is studied in a diamond anvil cell (DAC) with silicon oil as pressure medium by using angle dispersive XRD technology, and the EOSs of Cr3C2 under pressures up to 50 GPa were calculated based on DFT in this study.
The Cr3C2 (99.5%) samples was purchased from a company. Under ambient conditions, Cr3C2 has an orthorhombic structure (the space group is Pnam, as shown in Fig.
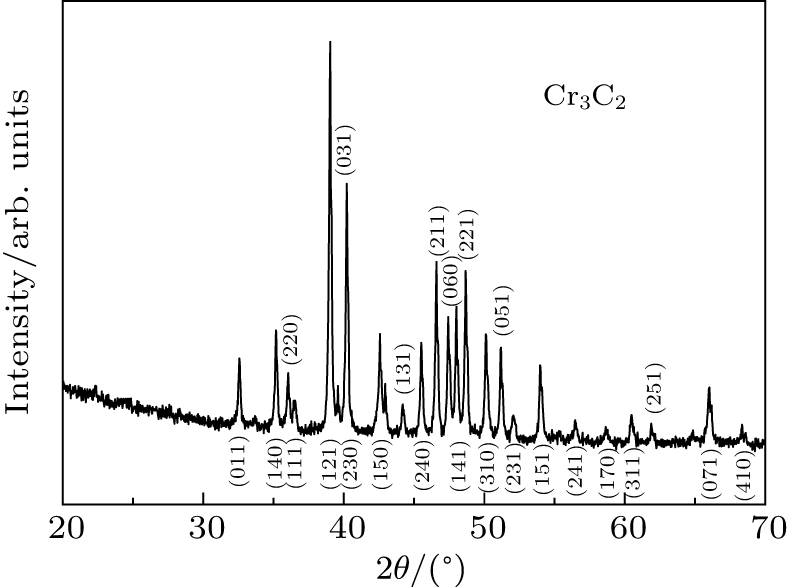 | Fig. 3. Representative powder XRD of Cr3C2 in ambient conditions with each peak marked by corresponding Miller indices, and x-ray wavelength λ = 1.5406 Å. |
The high-pressure XRD experiments of in-situ synchrotron radiation were carried out on 4w2 beam line of Beijing Synchrotron Radiation Facility (BSRF). PILATUS detector was used to receive the diffraction pattern, and CeO2 standard was used to calibrate the distance between the sample and the detector and the orientation of the detector. Each XRD pattern was obtained from the sample that has been exposed to the beam for 5 min–8 min. We used fit2d software[13] to process and analyze the XRD patterns.
The DFT calculations were carried out by using the Vienna ab-initio simulation package (VASP).[14] In addition, DFT calculations include structural optimization and enthalpy, in which the Perdew–Burke–Ernzerho[15] was applied to exchange–correlation functions. The interaction between the real electron and the valence electron of the ion was calculated by the projection affixed plane wave (PAW) method. The valence electrons of C and Cr were 2s22p2 and 3d54s1, respectively. Monkhorst pack method was applied to the Brillouin region integration of Cr3C2 system, and the integration grid was 8× 8× 8. The truncation energy of plane wave basis function was measured, and its convergence accuracy was less than 2× 10−3 eV, and the value of 600 eV was given. Cr3C2 had an orthorhombic structure (space group Pnam) with lattice parameters a = 5.4767 ×, b = 11.4621 Å, c = 2.7882 Å, and α = β = γ = 90° in ambient conditions. Using the third-order Birch–Murnaghan equation to fit the volume–pressure data, the bulk modulus (K0) and its first derivative (
The diffraction patterns obtained are analyzed with fit2d software.[13] The maximum pressure of the experiment is 44.5 GPa, and the pressure value is given by ruby sensor.[12] The diffraction patterns of Cr3C2 at different pressures are shown in Fig.
The lattice parameters and the equivalent unit cell volume of Cr3C2 under different pressures are fitted by d-spacings of d(011), d(101), d(121), d(230), d(151). The compressibility of a/a0, b/b0, and c/c0 in the orthorhombic structure of Cr3C2 is shown in Fig.
 | Fig. 5. Compressibility of lattice parameters of Cr3C2 and calculation result in generalized-gradient approximation (GGA). |
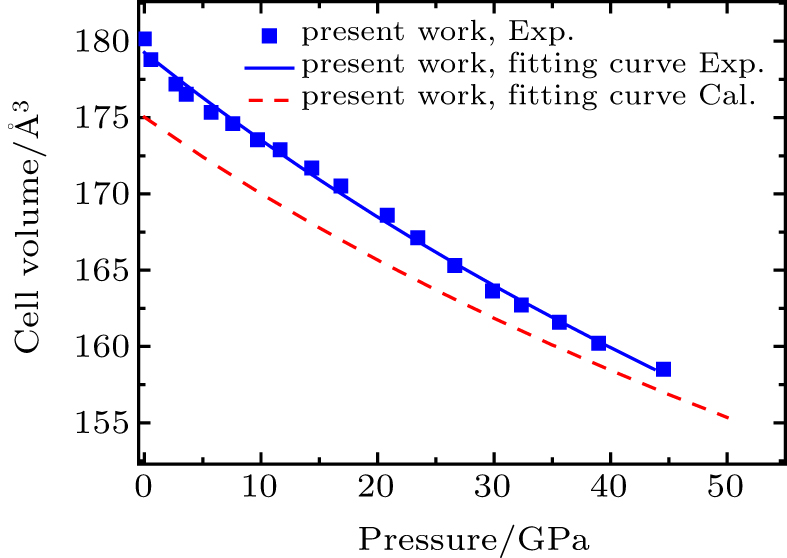
The unit cell volume of the compression curve of experimental result and GGA calculation result are shown in Fig.
The third-order Birch–Murnaghan equation is used to fit the change of cell volume with pressure. And we can obtain the K0 and 

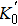
The bulk modulus and its first derivative with respective to pressure of the orthorhombic structure are 292(18) GPa and 3.25(0.85), respectively. The theoretical calculation result is 323(1) GPa. It can be seen that the experimental bulk modulus in present work is 10% lower than the GGA calculation result in present work. As the DFT transforms the real multi-electron problem into a single-electron problem to calculate single-electron effective potential, the total energy of electron gas is only a function of electron density, and the corresponding density is the ground state density of a single particle. The exchange correlation energy in the GGA approximation is related to density and the density gradient. In addition, the temperature of electrons in a real material in ambient conditions is not absolute zero compared with in the GGA approximation. Thus, the bulk modulus of Cr3C2 needs further studying. The values of bulk modulus (K0), its derivative with respect to pressure (
| Table 1. Values of bulk modulus (K0) and its derivative with respect to pressure ( |
We calculate the electronic structure of Cr3C2 at high pressure. Figure
In this work, we studied the EOSs of Cr3C2 in the modified Mao–Bell DAC under quasi-static compression at pressures up to 44.5 GPa by XRD at room temperature. The orthorhombic structure of Cr3C2 is stable at pressures up to the highest pressure of 44.5 GPa without phase transition. The bulk modulus of XRD is 292(18) GPa and its first derivative with respect to pressure is 3.25(0.85). The calculated bulk modulus is 323(1) GPa. The results show that the bulk modulus calculated in the GGA is slightly higher than the experimental results.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] |