† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Shandong Province, China (Grant No. ZR2014AM026).
The properties of one-photon absorption (OPA), emission and two-photon absorption (TPA) of a bipyridine-based zinc ion probe are investigated employing the density functional theory in combination with response functions. The responsive mechanism and coordination mode effect are explored. The structural fluctuation is illustrated by molecular dynamics simulation. The calculated OPA and emission wavelengths of the probe are consistent with the experimental data. It is found that the red-shift of OPA wavelength and the enhancement of TPA intensity are induced by the increased intra-molecular charge transfer mechanism upon metal binding. The structural fluctuation could result in the blue-shift of TPA wavelength and the decrease of the TPA cross section. The TPA properties are quite different among the zinc complexes with different coordination modes. The TPA wavelength of the complexes with two ligands is close to that of the probe, which is in agreement with the experimental observation.
In recent years, various kinds of two-photon (TP) fluorescent probes have been developed because of their important applications in TP fluorescence microscopy.[1–4] In comparison with one-photon microscopy, TP fluorescence microscopy could increase penetration depth, lower tissue auto-fluorescence and self-absorption, and reduce photon-damage and photo-bleaching. Therefore, TP fluorescence microscopy has become a critical tool in imaging of living cells and tissues and TP fluorescent probes has been extensively used to detect cation and anion ions,[5–8] pH values,[9,10] small molecules,[11,12] DNA,[13] and so on. The zinc ion is an important element in living body and takes part in many biological processes.[14–16] To detect the distributions of the zinc ions in the living systems is highly required. A number of turn-on or ratiometric TP probes for zinc ion detection have been synthesized and their bio-imaging applications have been explored.[5–7]
In contrast to plenty of experimental work, the theoretical study on the TP zinc ion probe is still very scarce in the literature.[17–24] Ren and co-workers studied a series of TP zinc ion probes based on the intramolecular charge transfer (ICT)[17–19] and photo-induced electron transfer (PET) mechanism.[20] The natural bond orbital (NBO) analysis was used to explore the ICT mechanism and the characteristics of molecular orbitals were calculated to illustrate the PET mechanism. Bednarska et al. elucidated the sensing mechanism for a bipyridine-centered ratiometric zinc ions probe employing the quantum chemical method in combination with molecular dynamics (MD) simulations.[21] The structures of the probe in water were obtained by a force-field MD approach. The average linear and nonlinear optical properties were calculated. In our previous work, the responsive mechanism of several TP zinc ion probes was investigated[22,23] and some new zinc ion probes were designed based on the structure-property relationships.[22] Special emphasis was placed on the effects of isomerism and coordination mode on the optical properties.[24]
Recently, Li et al.[25] synthesized a ratiometric TP fluorescence probe for sensing of zinc ions. The two-photon absorption (TPA) cross section of the probe is high and increases after coordination with the Zn2+. In order to understand the response mechanism of the probe, we perform a theoretical study on the one-photon absorption (OPA), emission and TPA properties of the probe before and after combination with the Zn2+ using density functional theory (DFT). The experiment molecular structures are analyzed by a potential energy surface scan. For a probe in solution at room temperature, the structural fluctuation and coordination mode effect are highly required to take into account. In this work, the structural fluctuation is illustrated by MD simulation and the structure-property relationship is examined. Besides, several coordination modes are considered and the effects on TPA properties are further explored at length. To the best of our knowledge, the coordination mode effects have not been investigated theoretically for TP metal ion sensors by other research groups. Our study will provide a good understanding of the experiment results for TP zinc ion probes.
In this study, the expressions for one- and two-photon absorption processes are the same as our previous work, such as the ones given in Refs. [22,26]. The TPA cross section can be obtained by calculating the individual TP transition matrix elements[27] and the matrix elements can be calculated through the single residues of the quadratic response function in response theory.[28] The calculation details are as follows. The optimization of ground state geometries is performed by using DFT with the 6-31G(d,p) basis set and the B3LYP hybrid functional. The OPA properties are computed by the time-dependent DFT (TD-DFT) approach at the CAM-B3LYP level with the 6-31G(d) basis set. The first excited state geometry and fluorescence properties are calculated using TD-DFT at the same level. All of the calculations mentioned above are carried out in the Gaussian 16 program.[29] The response theory which is embedded in the Dalton 2013 package[30] is employed for computing the TPA transition matrix and the CAM-B3LYP functional with the 6-31G(d) basis set are used. Moreover, the effect of the methanol solvent is taken into account implicitly using the polarizable continuum model in both Gaussian and Dalton calculations.
In the MD simulations, all of the parameters of molecular force field are taken from the general Amber force field (GAFF)[31,32] in the Amber 14 package.[33] As our previous work,[34,35] the partial atomic charges are obtained by fitting the electrostatic potential from the Hartree-Fock 6-31G(d) calculations for the probe and the solvent molecule. One probe and 1000 methanol molecules are contained in a simulation box. The simulations are performed by the Gromacs[36] program with a time step of 1fs and the Berendsen barostat and thermostat are used.
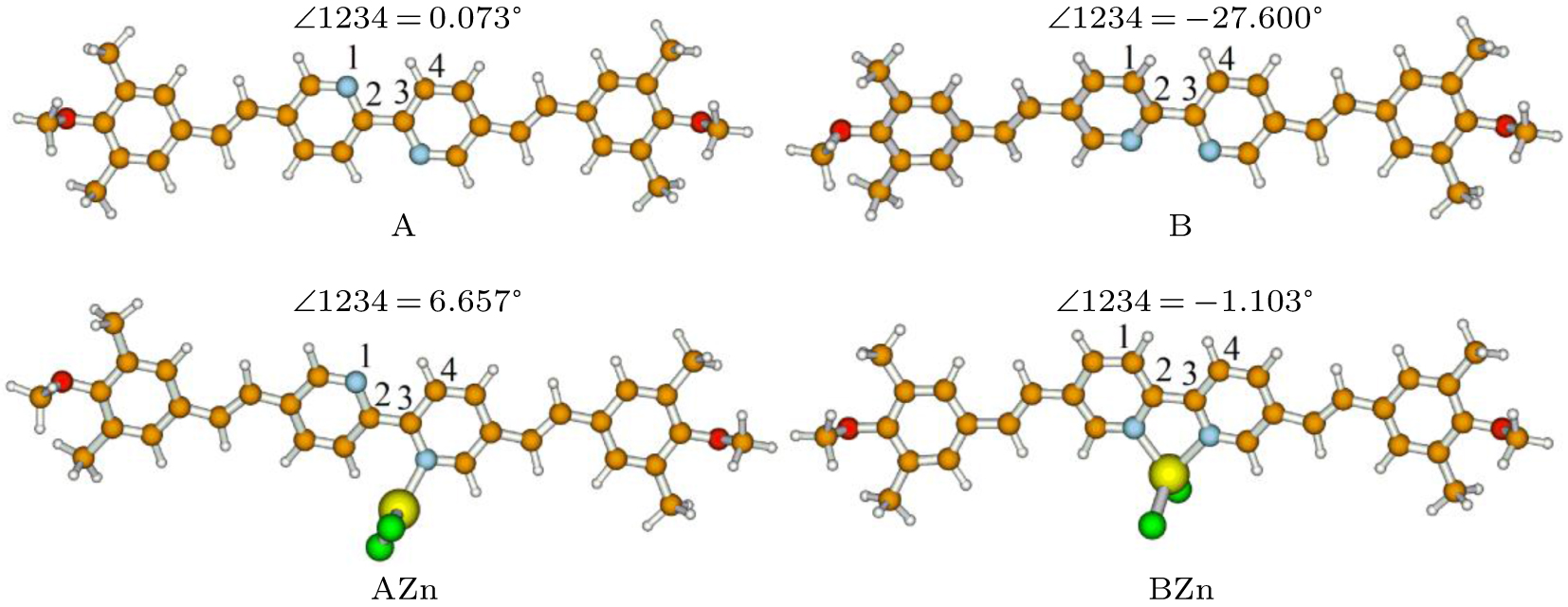
The chemical structure of the probe, named as A, is shown in Fig.
The energies of a set of the structures with different dihedral angles between the central two pyridine rings are calculated and the energy curves are shown in Fig.
In quantum chemical calculations, the temperature effects usually are not considered. For a molecule in solution at room temperature, the structural fluctuation becomes possible. In some cases, the structural fluctuation could produce important effects on spectra.[35] It is easy to find that the probe A has a very flexible structure because the dihedral angle between the two pyridine rings could be changed. We take the probe A as the initial conformation. Figure
The experimental linear absorption and emission wavelengths of the probe A are located at around 360 nm and 465 nm. After coordinating with the Zn2+ ions, the absorption wavelength shifts to 400 nm and the emission wavelength is observed at 550 nm.[25]
Table
The geometry for the first excited state has been optimized by the TD-DFT method at B3LYP level with 6-31G(d,p) basis set in methanol solvent. The obtained fluorescence emission wavelength λem, corresponding oscillator strength fem, and transition nature are also given in Table
In the experiment of TPA-induced fluorescent, the near-infrared laser is often employed as the excitation source. Its wavelength is near to two times of the OPA wavelength. The TPA-induced fluorescence intensity has a linear dependence on the square of the excitation intensity, which can be used to confirm that the spectra are mainly produced by the TPA process. Although TPA has different selection rules with respect to OPA, the TPA-induced fluorescent process is usually the same as the OPA-induced fluorescent process according to Kasha’s rule. The probe we studied is a ratiometric TP probe for zinc ion. For the ratiometric probe, the fluorescence quantum yield usually has considerable values (> 0.5) before and after combination with metal ions.
| Table 1. One-photon absorption and emission properties of the molecules. . |
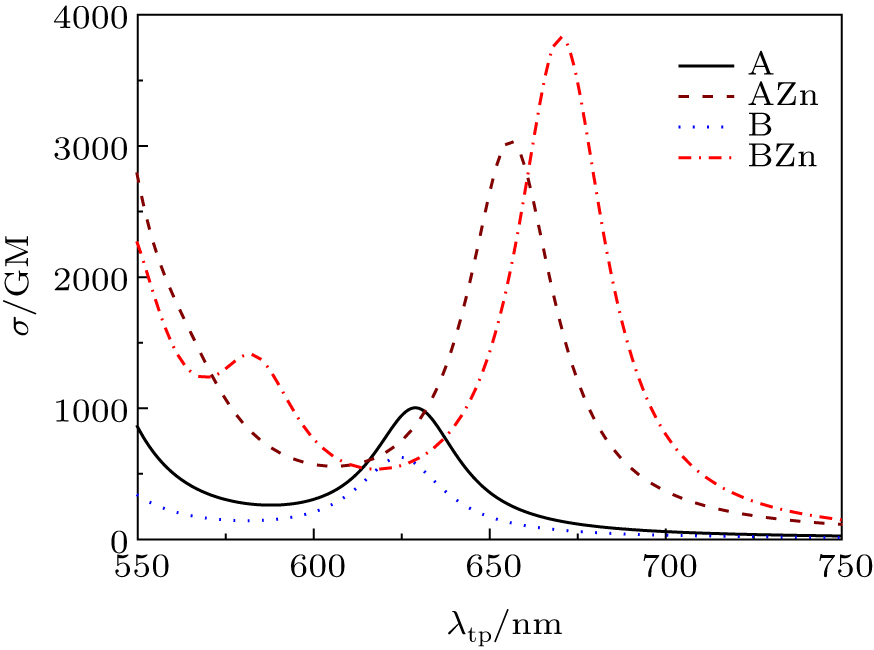
The TPA wavelength λtp and cross section σ of the six lowest excited states are presented in Table
| Table 2. Two-photon absorption wavelengths λtp and cross sections σ of the six lowest excited states of the molecules (A and B) and their corresponding zinc complexes. . |
For the probe A, the fourth excited state has the largest cross section σ with the value of 2077 GM at 530 nm. The second and sixth excited states also have considerable cross sections. The σ of the second excited state is 949 GM at 629 nm, which produces one absorption peak in the spectrum (see Fig.
As is well known, TPA properties are determined by the ICT process.[17,18,38–40] In order to obtain a better understanding of the ICT process, we have performed calculations of NBO charge analysis for the A and B, as well as their zinc complexes in the ground states and the first excited states. These molecules are divided into three parts X, Y, and Z, as shown in Fig.
| Table 3. Net charges (in units of e) for divided parts of the molecules in the ground states and the first excited states. . |
The QX0 and QX1 stand for the net charge of part X in the ground state and the first excited state, respectively. Δ QX represents the net charge difference of part X between the ground state and the first excited state. Similar to this definition, Δ QY and Δ QZ denote the net charge difference of part Y and part Z between the ground state and the first excited state. For the probe A, it is found that the net charge of part X in the first excited state QX1 is 0.105e, which is more electropositive than the value in the ground state QX0, 0.045e. The net charge of part Y in the first excited state (QY1, –0.210e) is more electronegative than in the ground state (QY0, –0.090e). The case of part Z is the same as part X. This indicates that part X and Z are the donors of the molecule and part Y is the acceptor of the molecule. For the AZn, part X and part Z also act as the donors and part Y is the acceptor. But the net charge differences of the two donors are different. Besides, the Δ QY is –0.178e, which means the charge transfer is much larger than that of the A. This demonstrates that the ICT is increased upon metal binding. The charge distribution of the probe B is similar to the case of the A. The Δ QY of the BZn is –0.282e, which means the BZn has the strongest charge transfer ability among these molecules. The ICT is also increased greatly after coordinating with the Zn2+ ion.
The structural fluctuation effects are considered and the TPA spectra for the structures with different dihedral angle φ based on optimized A conformation are computed and the results are given in Fig.
In the experiment, the TPA peaks are nearly at the same wavelength before and after combination with the Zn2+. However, the calculated TPA wavelength of the BZn is red-shifted greatly with respect to the A. Even after considering the structural fluctuation effects, the discrepancy still exists. And also, besides the coordination mode of the AZn and BZn, it is possible that the zinc ion could coordinate with two ligands. Then three new coordination modes AAZn, ABZn and BBZn are taken into account and their structures are shown in Fig.
 | Fig. 8. Chemical structures and the corresponding optimized geometries of the zinc complexes AAZn, ABZn, and BBZn. |
The TPA wavelength λtp and cross section σ of the ten lowest excited states for the three complexes are presented in Table
| Table 4. Two-photon absorption wavelengths λtp and cross sections σ of the ten lowest excited states of the zinc complexes AAZn, ABZn, and BBZn. . |
The OPA, emission and TPA properties of a bipyridine-based TP fluorescent probe before and after combination with Zn2+ are theoretically investigated at a DFT level. The structural fluctuation is illustrated by MD simulations. The ICT mechanism is specified. Several coordination modes are considered and the effects on TPA properties are explored at length. The calculated OPA and emission wavelengths of the probe are consistent with the experimental data. The OPA wavelengths show red-shifts and the TPA cross sections are enhanced greatly due to the increased ICT mechanism upon metal binding. It is found that the structural fluctuation could result in the blue-shift of TPA wavelength and the decrease of TPA intensity. The TPA wavelength and cross section are quite different among the zinc complexes, which demonstrates the significant coordination mode effects on TPA. It is interesting to find that the TPA wavelengths of the complexes with two ligands are close to that of the probe, which could produce the experimental observations.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] |