† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 51371010, 51572066, and 50801002), the Beijing Municipal Natural Science Foundation, China (Grant No. 2112007), the Fundamental Research Funds for the Central Universities (Grant No. PXM2019-014204-500032), and the Science Fund from the Advanced Space Propulsion Laboratory of BICE and Beijing Engineering Research Center of Efficient and Green Aerospace Propulsion Technology, China (Grant No. LabASP-2018-09).
Mg3Sb1.5Bi0.5-based alloys have received much attention, and current reports on this system mainly focus on the modulation of doping. However, there lacks the explanation for the choice of Mg3Sb1.5Bi0.5 as matrix. Here in this work, the thermoelectric properties of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) compounds are systematically investigated by using the first principles calculation combined with experiment. The calculated results show that the band gap decreases after Bi has been substituted for Sb site, which makes the thermal activation easier. The maximum figure of merit (ZT) is 0.27 at 773 K, which is attributed to the ultra-low thermal conductivity 0.53 W·m−1·K−1 for x = 0.5. The large mass difference between Bi and Sb atoms, the lattice distortion induced by substituting Bi for Sb, and the nanoscale Bi-rich particles distributed on the matrix are responsible for the reduction of thermal conductivity. The introduction of Bi into Mg3Sb2-based materials plays a vital role in regulating the transport performance of thermoelectric materials.
Thermoelectric devices have received much attention due to the fact that the solid-state conversion process has no mechanical transmission parts, quiet, and friendly to environment.[1–5] Thermoelectric conversion efficiency is the decisive factor to expand the application of thermoelectric technology. The conversion efficiency is critically related to the dimensionless figure of merit, ZT = α2T/(ρκ), where α is the Seebeck coefficient, T is the absolute temperature, ρ is the electrical resistivity, and κ is the thermal conductivity of the thermoelectric materials.[6] Satisfactory ZT will be achieved with low thermal conductivity and favorable electrical transport properties, which can be realized in the so-called phonon–glass–electron–crystal (PGEC) materials. Zintl phase compounds are ideal candidates for the thermoelectric materials due to their complex structure conforming to PGEC concept.[7–14]
The Mg3Sb2-based thermoelectric materials are typical zintl compounds, which consist of nontoxic, earth abundant, and inexpensive elements and have received much attention.[15–19] Strategies for enhancing ZT mainly focus on two directions: increase the power factor (PF = α2/ρ), and reduce the lattice thermal conductivity. Band engineering approaches, such as carrier filtering effect and modulation doping, are employed to optimize the power factor.[20–22] Phonon scattering mechanisms, including defect engineering and nanostructuring, are employed to reduce the lattice thermal conductivity.[23,24] Among the reported Mg3Sb2-based thermoelectric materials, single p-type doping, such as Li,[25] Na,[26] Ag[27] in Mg site, and Pb,[28] Bi[29] in Sb site, can enhance the electrical performances through increasing the carrier concentration. The highest power factor 7.5 μW·cm−1·K−1 was obtained in Na-doped Mg3Sb2 alloys.[26] Compared with p-type Mg3Sb2-based materials, n-type doping is very efficient to enhance the thermoelectric performance, especially in Mg3Sb1.5Bi0.5-based compounds. Shi et al.[30] and Imasato et al.[31] have investigated La doping in Mg site, and Se,[19] Te[16] doping in Sb site to optimize the thermoelectric performances of Mg3Sb1.5Bi0.5-based materials. Among them, Te-doped Mg3Sb1.5Bi0.5 owns the highest ZT of 1.65 at 725 K.[16] However, the role of Bi in Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) still lacks systematic investigation.
Here in this work, the effects of Bi on electrical and thermal transport performance in Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) compounds are studied by combining the theoretical study with experimental research. The electronic structures of pure and Bi-doped Mg3Sb2 are calculated based on the density functional theory (DFT). Bulk Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples are synthesized by combining the suspension induction melting method with spark plasma sintering (SPS) method. The phase, microstructure, and thermoelectric properties of the samples are analyzed.
Density function theory (DFT) calculations of pure and Bi-doped Mg3Sb2 were carried out on the Cambridge Serial Total Energy Package (CASTEP). The band structures of undoped and Bi-doped Mg3Sb2 were calculated in a 2 × 2 × 1 supercell by the generalized-gradient approximation of Perdew–Burke–Ernzerhof (GGA-PBE) functional for comparison. The plane-wave cut off energy was set to be 700 eV, and the Brillouin zone k-point mesh density was 4 × 4 × 4. All samples were synthesized by combining the suspension induction melting method with spark plasma sintering (SPS) method. Magnesium grains (99.9 wt%, Alfa Aesar), antimony chunks (99.99 wt%, Alfa Aesar), and bismuth pieces (99.999 wt%, Alfa Aesar) were weighed according to the nominal compositions of Mg3Sb2−xBix (x = 0.4, 0.45, 0.5, 0.55), which will sustained suspension induction melting three times. The graphite die loaded with powder, obtained from crushing the melted ingot, was sintered by SPS at 953 K under a pressure of 50 MPa for 10 min.
The x-ray diffraction (XRD) analysis of the sintered Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples was performed on DMAX-IIIB equipped with Cu anode target (Kα ray, λ = 1.5418 Å). The microstructure of fresh fractured surface with x = 0.5 was observed on filed-emission scanning electron microscopy (SEM, FEI NANO200). The transmission electron microscopy (TEM, Tecnai F30) and high angle annular dark field scanning transmission electron microscope (HAADF-STEM) were employed to investigate the detailed microstructure features. The actual compositions of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples were confirmed by x-ray fluorescence spectrometer (XRF, XRF-1800). Carrier concentration and mobility were measured on a physical property measurement system (PPMS, Accent HL5500 Hall System) at room temperature. The measurement of electrical resistivity (ρ) and Seebeck coefficient (α) were carried out in a helium atmosphere (ULVAC ZEM-2). Thermal conductivity (κ = λ Cpd) was calculated by using the measured thermal diffusivity (λ) and specific heat (Cp) by laser thermal conductivity detector (ULVACTC-7000), density (d) by Archimedes method.
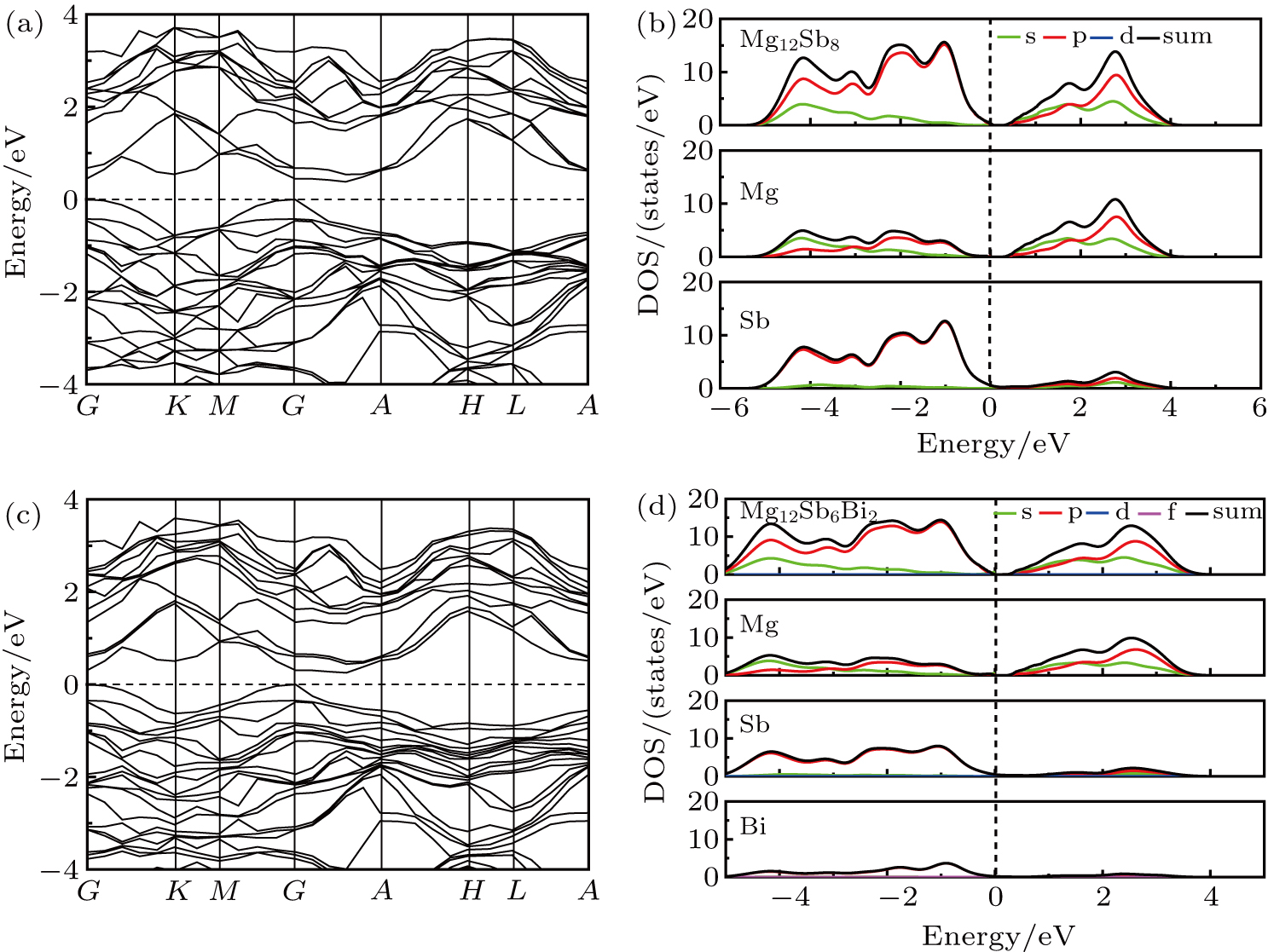
The calculated electronic band structure and density of states (DOS) of Mg12Sb8 and Mg12Sb6Bi2 are shown in Fig.
The XRD patterns of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples with α-La2O3 (space group P-3m1, No. 164) are shown in Fig.
The SEM image of the fracture parallel to the pressing direction of Mg3Sb1.5Bi0.5 sample is shown in Fig.
To identify the doping efficiency of Bi in Mg3Sb2, the physical parameters of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) alloys are measured on PPMS at room temperature and the results are listed in Table
| Table 1. Room-temperature Hall measurement data and XRF composition of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples. . |
Figure
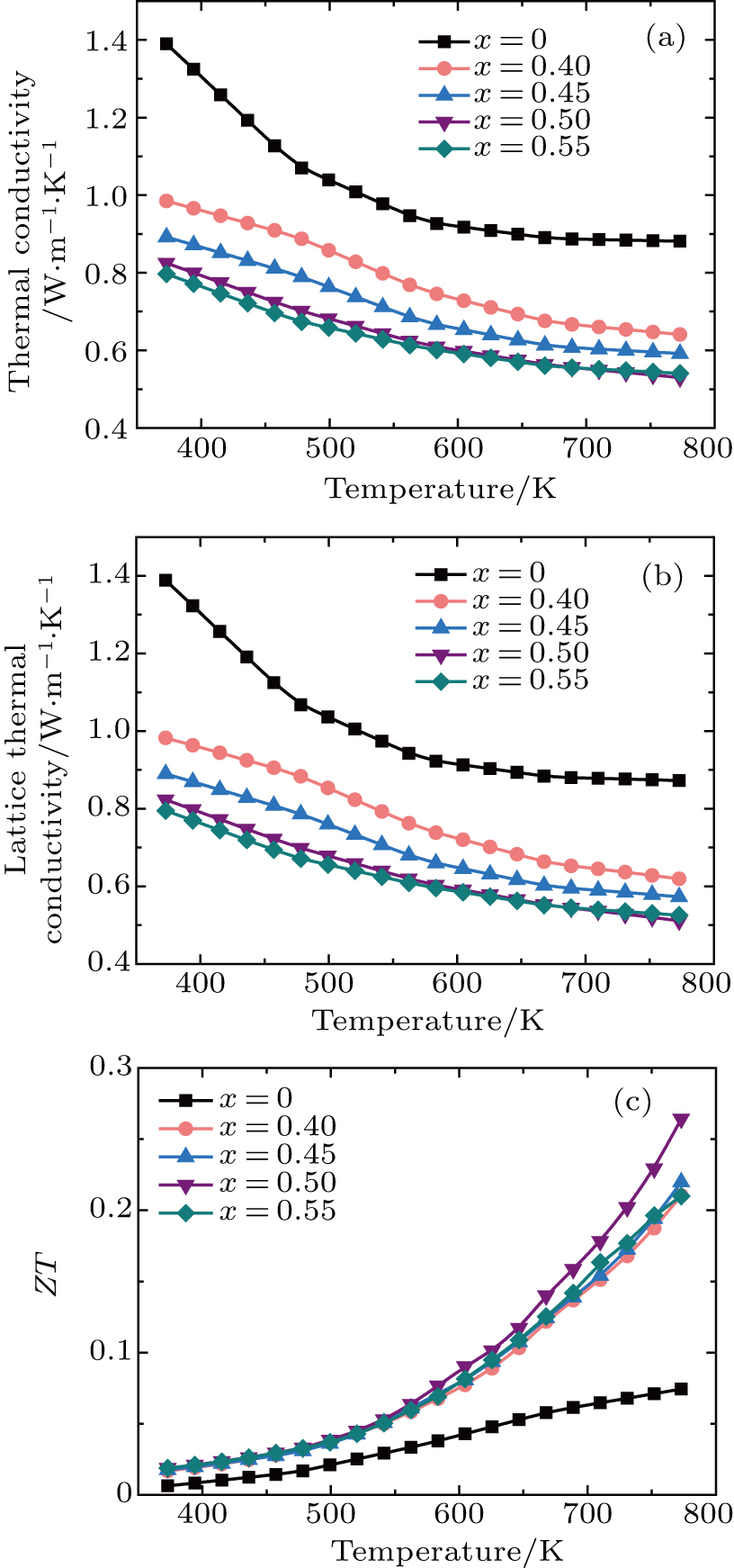
The temperature dependence of total thermal conductivity of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples is displayed in Fig.
The dimensionless figures of merit ZT of Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) compounds are shown in Fig.
In summary, according to the first-principles calculation results, the band gap decreases when Bi is substituted for Sb site, which is conductive to the improvement of carrier concentration. The Mg3Sb2−xBix (0.4 ≤ x ≤ 0.55) samples are successfully synthesized through combining the suspension induction melting method with the SPS method. A peak ZT of 0.27 is obtained at 773 K for x = 0.5. In comparison with that of other single doping Mg3Sb2 materials, the thermoelectric performance of Bi-doped samples is improved primarily by reducing the thermal conductivity, which is due to the enhanced scattering for phonons from the heavier Bi atoms substituted for Sb site and the nanoscaled particles on the matrix. The ultra-low thermal conductivity makes the Mg3Sb1.5Bi0.5-based materials receive much attention for the applications in thermoelectric devices.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] |