† Corresponding author. E-mail:
Project supported by the National Key Research and Development Program of China (Grant No. 2017YFA0402300).
The direct Coulomb explosion of N2O2+ has been investigated experimentally after double-ionization by a single extreme ultraviolet (EUV) photon with an energy of ∼ 38.5 eV. From the ion–ion time-of-flight coincidence spectrum, the de-nitrogenation (N2O2+ → N+ + NO+) and de-oxygenation (N2O2+→ O+ + N2+) photodissociation channels of N2O2+ are unequivocally identified. The measured kinetic energy release (KER) distribution of the de-nitrogenation channel presents a major peak accompanied by a shoulder structure. We find that the major peak can be attributed to the direct photodissociation of the 11Δ and 11Σ+ states, while the shoulder structure should be ascribed to the predissociation of the 11Σ and 11Σ+ states via 13Π state.
N2O is very stable in the troposphere and its global warming potential is about 300 times higher than that of CO2.[1] Even worse, after transported to the stratosphere, N2O can release active nitrogen oxides (NO) via the de-nitrogenation process induced by the solar radiation or charged particles from the space.[2] There, NO catalytically destroys the ozone layer. In fact, it is found that the agricultural practices and the use of N-fertilizers have already greatly enhanced emissions of N2O for the last two decades[3] which is becoming the largest ozone-depleting emission in the 21st century.[2] Studies on the de-nitrogenation processes of N2O in presence of external fields are thus of significance in the air pollution control and attract more and more attentions.[4,5]
A non-negligible de-nitrogenation channel originating from the Coulomb explosions of N2O2+ has been reported recently.[5,6] After removal of two electrons from N2O induced by the solar radiation or charged particles from the space, the molecular dication N2O2+ subsequently dissociates through four different fragmentation channels as follows:[6–9]
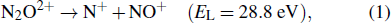



The KER of the de-nitrogenation channel of N2O2+ induced in collisions with electrons,[7,10–12] highly charged ions (HCIs),[8,13] and intense infrared laser fields[5,14–16] have been extensively studied. Generally, the dissociation energy strongly depends on the molecular potential curves populated. Accordingly, the KER distributions are subject to projectile species and energies, and a large variety of spectra have been reported in literature.
In 1991, Eland and Murphy found that the peak of KER is around 7.2 ± 0.7 eV induced by 1300 eV electron impact.[10] At a much lower collision energy of 50 eV, the KER was found to be at 6.3 ± 1.0 eV.[11] For higher electron impact energies of 10 keV, two peaks in the KER distribution were identified.[7] These peaks are located at 4.0 ± 0.5 eV and 5.3 ± 0.6 eV with relative intensities of 58:42, respectively. Later, similar structures were also observed for 5 keV electron impact where the major peak was found at 6.6 ± 0.2 eV.[12]
After double ionization of N2O molecules in intense infrared laser fields, the KER has been reported to be around 6.5 ± 2.0 eV,[14] 6.8± 2.0 eV,[15] and 6.8 eV[5] for different laser intensities. After absorptions of the single photons from the He II radiation (He IIα 30.4 nm, He IIβ 25.6 nm, etc.), the peaks were found to be centered around 6.4 ± 0.3 eV[17] and 6.3 ± 0.3 eV.[6] However, a different value of 7.2 ± 0.4 eV was reported for the shorter wavelength of 25.6 nm.[10] The dependence of the total kinetic energy of the de-nitrogenation process on the photon energy of synchrotron radiations was reported by Alagia et al.[18–20] It was also found that the photodissociation processes of N2O2+ are dominated by the direct Coulomb explosion in the case of photon energies between ∼ 35.5 eV and ∼ 38.5 eV.[21]
While these studies generally contribute to our understanding of the dissociation dynamics, in the case of the double ionization by electrons, ions, and intense infrared laser fields, various states of N2O2+ can be populated which can hardly be resolved in the KER spectra. Moreover, several electronic dissociation pathways are involved which can interfere, making an interpretation of the observed spectra even more complicated.
Very recently, the dissociation pathways of N2O2+ induced by collisions between 5.7 keV/u Xe15+ and N2O were distinguished.[8] In the work, the KER spectra showed some similarities to those of electron/photon impact, and the peak positions were found at 6.8 eV and 9.0 eV, respectively. It was concluded that the measured peak at 6.8 eV originates from two states: (a) the N2O2+ ground state (X3Σ−) which dissociates to N+(3P) + NO+(1Σ+); (b) the metastable state (13Π) which decays radiatively to X3Σ− and dissociates subsequently. The signature of the metastable (13Π) state could clearly be recognized from the long tail in the two-fragment time-of-flight coincidence spectrum.[21,22] A shoulder around 9.0 eV was attributed to N2O2+ (11Π, 11Σ−) dissociating to N+ (1D) + NO+(1Σ+) via the potential energy curve crossing of the 11Π and 11Σ− states of N2O2+
In this paper, we report on the direct Coulomb explosion of N2O2+ induced by the extreme ultraviolet photons at the energy of ∼ 38.5 eV. In our study, the photon energy is selected right below the metastable threshold energy (Emth = 38.5 eV[22]), and therefore, the population of the metastable state (13Π) is effectively suppressed. Meanwhile the molecular ion can only be populated in a few states, which greatly simplifies the analysis of the dissociation pathways. The momenta of the fragment ions are recorded in coincidence with the laser pulse by employing the reaction microscope for each fragmentation event. In the time-of-flight (TOF) coincidence spectra of two fragments, different dissociation channels of N2O2+ are resolved. The KER distributions of the de-nitrogenation channel and the corresponding dissociation pathways are discussed in detail.
The experimental setup is shown in Fig.
 | Fig. 1. Schematic view of the experimental setup (we note that the electron detector is not activated in the present work). |
In the reaction chamber, the monochromatic EUV pulse is focused onto the target. The gas target is prepared with the N2O gas of about 99.99 % purity using the supersonic gas target method, where the stagnation pressure is set to 12 bars.[27] Meanwhile, the background pressure in the target chamber of reaction microscope[28–30] can be kept at a sufficiently low value of 10–9 mbar. After absorption of the EUV photon, one or two electrons are removed from N2O molecule which dissociates into charged fragments due to the Coulomb repulsion. A uniform electrostatic field of ∼ 105 V/cm is used to project the positively charged fragments to the recoil ion detector (see Fig.
In the following discussion, the coordinate system is defined as following. The EUV light propagates in the Z direction, while the supersonic jet moves in the Y direction which is also the polarization direction of the EUV photons. Accordingly, the X direction is along the TOF spectrometer axis.
Figure

Figure
The observed difference can be ascribed to the collisional population of the metastable 13Π state in the previous studies,[21,22] a channel which is energetically not allowed in our experiment. In the present work, we focus on the processes of two electrons removed from the outer-shell Π-orbital of N2O by absorption of a ∼ 38.5 eV photon. The only accessible electronic states of the N2O2+ dication are the X3Σ−, 11Δ, and 11Σ+ states (see Fig.
 | Fig. 3. (a) The potential energy curves of N2O2+ given for the de-nitrogenation channel.[22] Vertical dashed lines indicate the Franck–Condon region. (b) The KER spectra of the de-nitrogenation process. The solid circles are experimental data, and the red solid lines are the fitting lines. The inset highlights the contributions from different pathways to the shoulder formation (see text). |
The KER distribution of the N2O2+ → N+ + NO+ dissociation channel is shown in Fig.
| Table 1. The KER peak values for N2O2+ → N+ + NO+ dissociation channel obtained in the present experiment and earlier reported experiments. . |
| Table 2. The KER values of de-nitrogenation channel derived from different dissociation pathways. . |
From the fitting results, we conclude that the major peak centered at 6.4 eV can be attributed to two processes: (i) the dominant photodissociation peak centered at 6.3 eV (black solid curve) which represents a direct decay from the 11Δ state to the second dissociation limit of N+ (1D) + NO+ (1Σ+), (ii) the second peak at 6.8 eV (green solid curve) is assigned to the direct dissociation of the 11Σ+ state to the same dissociation limit (L2). Meanwhile, the contribution from the ground state X3Σ− (blue solid curve, see Fig.
Accordingly, the shoulder structure around 8.8 eV can be interpreted as follows. As pointed out above, with ∼ 38.5 eV photon energy the electronic states of N2O2+ are mainly populated to the 11Δ and 11Σ+ states. Therefore, there is a good reason to believe that the shoulder structure is ascribed to the decay of the 11Δ and 11Σ+ states of N2O2+ to the first dissociation limit of N+ (3P) + NO+ (1Σ+). This dissociation takes place through the potential curve crossings with the 13Π state at C and D shown in Fig.
However, our results are different from the earlier results of Chen et al.[8] In their study, they used 5.7 keV/u Xe15+ beam ions to produce N2O2+, therefore, they observed a shoulder structure situated at 9.0 eV of the de-nitrogenation channel. This structure was explained by the population of the 11Π state in N2O2+ which predissociates to the second dissociation limit (L2) of N+(1D) + NO+(1Σ+). This predissociation occurs through two crossing points of the potential energy curves, as shown by A and B in Fig.
The Coulomb explosion of N2O2+ is studied with the reaction microscope double photoionization from N2O by extreme ultraviolet photons at ∼ 38.5 eV. The de-nitrogenation as well as the de-oxygenation channels are identified from the time-of-flight coincidence spectra. It is found that, in the de-nitrogenation channel, the N2O2+ dications mainly dissociate from the 11Δ and 11Σ+ states. In the KER spectra, a main peak centered at 6.4 eV with a shoulder towards higher energies is observed. Further studies show that the major peak is derived from the direct dissocaition of N2O2+ from the 11Δ and 11Σ+ potential curve to N+(1D) + NO+(1Σ+) (the second dissociation limit L2). The shoulder is originated from the dissociation of N2O2+ from the 11Δ and 11Σ+ states to N+(3P) + NO+(1Σ+) (the first dissociation limit L1) via the crossing of the 13Π state potential energy curves. Our work shows that the table-top monochromatic EUV beam line provides great advantages in detailed studies of the electronic states and dissociation dynamics involved in photo-ionization of molecular systems.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] |


