† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation for Joint Fund Key Project of China (Grant No. U1865206), the National Science and Technology Major Project of China (Grant No. 2017-VII-0012-0107), the National Defense Science and Technology Key Laboratory Fund of China (Grant No. 614220207011802), and the Key Area Research and Development Program of Guangdong Province, China (Grant No. 2019B090909002).
Nitrogen-doped TiO2 nanotubes (TNTs) were prepared by ion implantation and anodic oxidation. The prepared samples were applied in photocatalytic (PC) oxidation of methyl blue, rhodamine B, and bisphenol A under light irradiation. To explore the influence of doped ions on the band and electronic structure of TiO2, computer simulations were performed using the VASP code implementing spin-polarized density functional theory (DFT). Both substitutional and interstitial nitrogen atoms were considered. The experimental and computational results propose that the electronic structure of TiO2 was modified because of the emergence of impurity states in the band gap by introducing nitrogen into the lattice, leading to the absorption of visible light. The synergy effects of tubular structures and doped nitrogen ions were responsible for highly efficient and stable PC activities induced by visible and ultraviolet (UV) light.
Access to clean drinking water was recognized as a human right according to UN regulations in 2010.[1] However, due to human activities, the water pollution arising from organic compounds is growing steadily around the world.[2] To solve this environmental challenge, the technology of photocatalytic (PC) remediation of wastewater has been attached a great deal of attention because which is an effective method to deal with those recalcitrant organic pollutants.[2–5] TiO2 is the most useable photocatalyst for solving contamination problems on account of its excellent properties, such as low cost, nontoxicity, long durability, and chemical stability.[6,7] The PC process of TiO2 materials begins with the generation of photogenerated hole and electron (h+–e−) pairs on the surface under ultraviolet (UV) light irradiation. Then the h+ interacts with adsorbed organic compounds and H2O to produce hydroxyl radicals (⋅OH)) which can oxidize organic molecules dissolved in the water. Meanwhile, e− photoexcited to conduction band (CB) reacts with absorbed oxygen molecules. As a result, superoxide radical anions (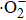
TiO2 only shows obvious PC activities excited by UV energy owing to its wide band gap (BG, 3.2 eV for anatase). In comparison with visible light (45%), UV light is merely a small fraction (5%) of the solar spectrum.[14] Therefore, numerous approaches have been applied to modify optical response of TiO2 from UV to visible light by reducing the threshold energy barrier, such as doping with some anions (C, S, F, B, P, and N) to reduce the BG energy of TiO2.[15–18] So far, nitrogen-doped TiO2 materials have been utilized in many PC applications.[19–21] It is convinced that the incorporation of nitrogen ions into the TiO2 crystal lattice can take place easily due to comparable size compared to oxygen atoms.[20,21] As a result, the doped TiO2 with reduction of BG shows enhancement of PC activities under visible light irradiation.[3,20]
The properties of nitrogen-doped TiO2, such as particulate morphology, BG, PC activities, doping sites, and amounts, extremely depend on the synthetic method.[8,22] It has been proposed in many studies that the lattice nitrogen atoms are beneficial to the visible light absorption, while several researchers attributed BG narrowing to NOx and NHx adsorbed on the surface.[20,22] Thus, the controversy about the dopant nature and electronic structure of nitrogen-doped TiO2 still remains. As a typical engineering process, ion implantation is usually employed to modify the chemical and physical properties of semiconductor including TiO2.[23–27] Compared to other doping methods, the merits of ion implantation are that not any element other than the interested one is involved, and a more reliable and much cleaner approach for introducing selected impurities into desired materials is offered. Nitrogen ions were introduced into TiO2 films by the ion implantation in our previous work,[28] and the direct bombardment of TNTs by this method with high energy (60 keV) was also reported.[25] The purpose of this work is to enhance the wastewater remediation abilities of TNTs observably under visible and UV light illumination, and clarify the mechanism of visible and UV light response of nitrogen-doped TNTs. Therefore, we propose a novel method in which nitrogen ions are doped into Ti foils by ion implantation, and then the wastewater remediation abilities of nitrogen-doped TNTs are evaluated by PC method. MB, RhB, and bisphenol A (BPA) are chosen as dyes and chemical respectively. To analyze the mechanism of improved PC activities of nitrogen-doped TNTs by electronic and band structure, calculations about nitrogen-doped anatase are also carried out using density functional theory (DFT).
Nitrogen ions are introduced into Ti foils with 60-keV ion energy and 10-mA/cm2 current density using an implanter equipped with a Kaufman ion source. The chamber pressure prior to implantation is kept at a value as low as 10−4 Pa, and then retaining the pressure at 10−2 Pa during ion implantation with a nitrogen flow rate of 6 sccm. The implanted doses are chosen as 1 × 1017 ions/cm2, 5 × 1017 ions/cm2, and 1 × 1018 ions/cm2. TNTs and nitrogen ions-doped TNTs are produced by the method of anodization using unimplanted and implanted Ti foils as anode respectively. During the anodization, a two-electrode system with platinum mesh as cathode and Ti foil and implanted Ti foils as anode are used at room temperature. The anodization is carried out in an electrolyte composed of ethylene glycol with 1-wt% water and 3-wt% NH4F. The samples are anodized for 120 min with 50-V constant voltage using a DC power supply. Lastly, the anodized samples are dried in air (80 °C for 2 h) after being rinsed with deionized water, and then are calcined at 450 °C for 4 h with heating rate of 5 °C⋅min−1 in the ambient atmosphere. Those samples are marked as N-1-TNTs, N-5-TNTs, and N-10-TNTs corresponding to the implanted TNTs with doses of 1 × 1017 ions/cm2, 5 × 1017 ions/cm2, and 1 × 1018 ions/cm2 respectively.
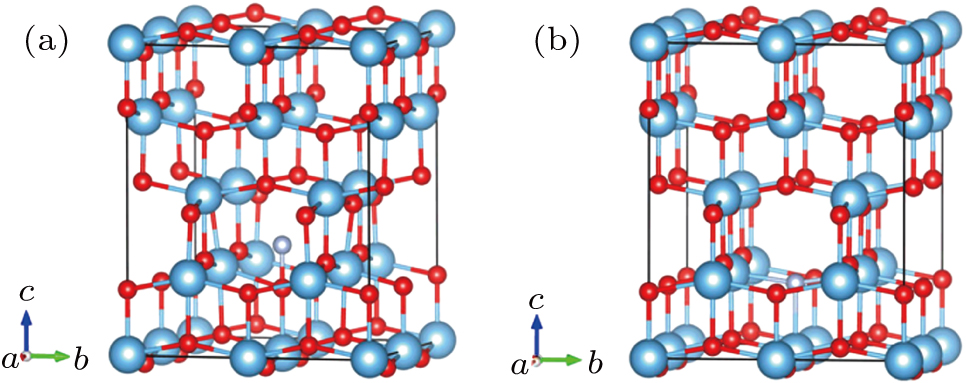
The DFT[29] calculations are carried out using the projector augmented wave (PAW) pseudopotentials-based Vienna ab initio simulation package code (VASP),[30] in which the exchange–correlation potential is the local density approximation (LDA). The values of onsite Coulomb interaction U within LDA for Ti 3d and O 2p states are 4.37 eV and 7.51 eV respectively.[31,32] As for N 2p states, the U value is selected as zero. Monkhorst–Pack k-point meshes of 4 × 4 × 4 are chosen for electronic property calculations. The electronic states are expanded using plane wave basis set with a cutoff energy at 450 eV. The energy at 5 × 10−6 eV is set as the convergence threshold for self-consistent field energy, and the atomic relaxations are performed until the residual forces are below 0.01 eV/Å. The (101) plane is chosen in the calculation because which is the dominant peak of TNTs according the results of experiment.[9–13] The two modeled anatase (101) nitrogen doping structures are shown in Fig.
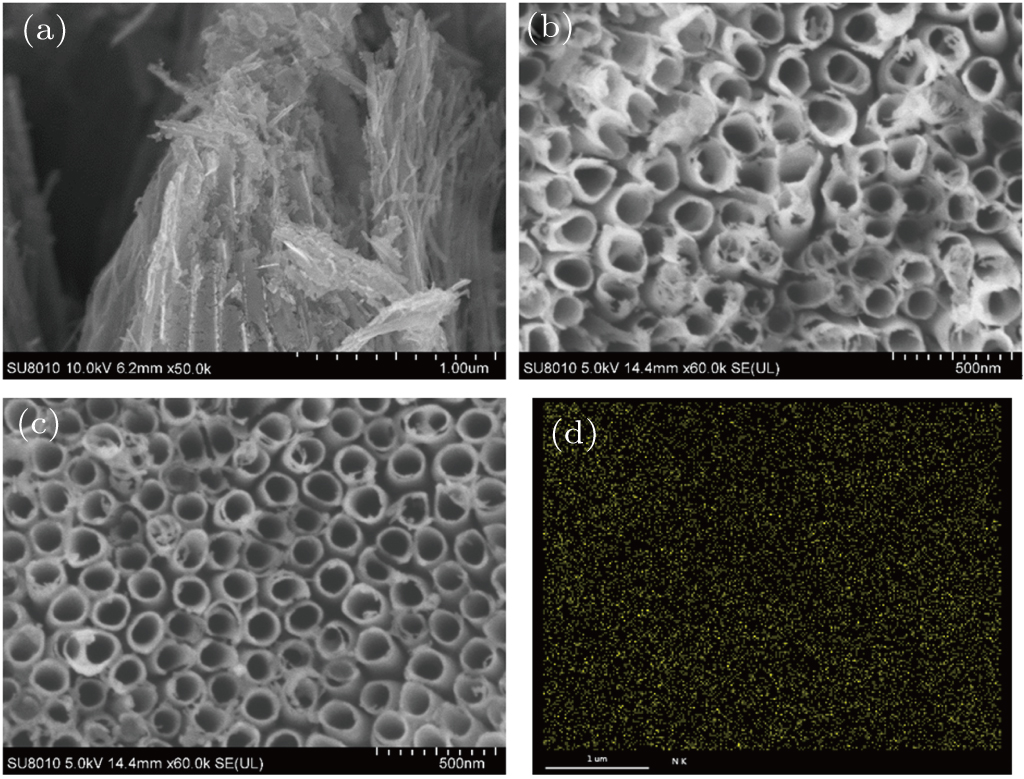
The morphologies of prepared samples are determined by field emission scanning electron microscope (FESEM, Hitachi SU8010) equipped with energy dispersion spectrometer (EDS). For the PC degradation experiments, MB, RhB, and BPA are selected. The initial concentrations are 80 mg/L for dyes and 50 mg/L for BPA respectively. For dissolution of BPA in water, the mixture is dealed with ultrasonic waves for 3 h. Before PC degradation, the TNTs and nitrogen-implanted TNTs (1 cm × 2 cm) are dipped in the solutions for 30 min to establish the equilibrium between adsorption and desorption. During PC degradation, all the samples are immersed in 3-ml solutions, and irradiated by visible and UV light. At given intervals, the photocatalysts are taken out and the solutions are analyzed using a UV-Vis spectrophotometer (Shimadzu UV-3600). After analysis, the photocatalysts are put into the reactor again for PC degradation.
The wavelengths used in absorbance measurements are 664 nm, 553 nm, and 277 nm for MB, RhB, and BPA, respectively. The removal efficiency of compound is examined according to the ratio of Ct and C0, where Ct and C0 are the absorbance of compound aqueous solution at time t and 0 at the analytical wavelength, respectively. The selected 365-nm UV light illumination is performed using a 500-W high-pressure mercury lamp. A 300-W tungsten–halogen lamp is chosen as the visible light source equipped with a cut-off filter with edge at 420 nm to remove UV light. The UV light intensity on the surface of samples is measured to be 213 μW/cm2 by UV irradiance meter (UV-A, BNU), while the flux of the visible light is found to be 10710 lx measured by illumination photometer (ST-85, BNU).
Figure
The band structure and density of states (DOS) of pure TiO2 are calculated, which are shown in Fig. S2. From the results, it can be seen that pure TiO2 is an indirect-gap semiconductor because the M and G points are corresponding to VB maximum and CB minimum respectively. The minimum BG width between VB maximum and CB minimum is about 3.21 V, which is in accordance with the experimental result of 3.2 eV. It is obviously that the VB of pure TiO2 is dominated by the O 2p states and the CB is mainly contributed by the Ti 3d states. The doped nitrogen atom not only affects the crystal structure of TiO2, but also influences its electronic structure and properties. For the cases of both I–N- and S–N-doped TiO2, the doped nitrogen atoms introduce four new states of N 2p orbitals in the BG. However, the new states locate at different positions. For the case of S–N, the new states are closer to VB maximum more, and connect with VB maximum in some regions. While the new states appear in the BG only to form impurity energy level for the case of I–N. So, S–N can reduce the BG width of TiO2. As shown in Figs.
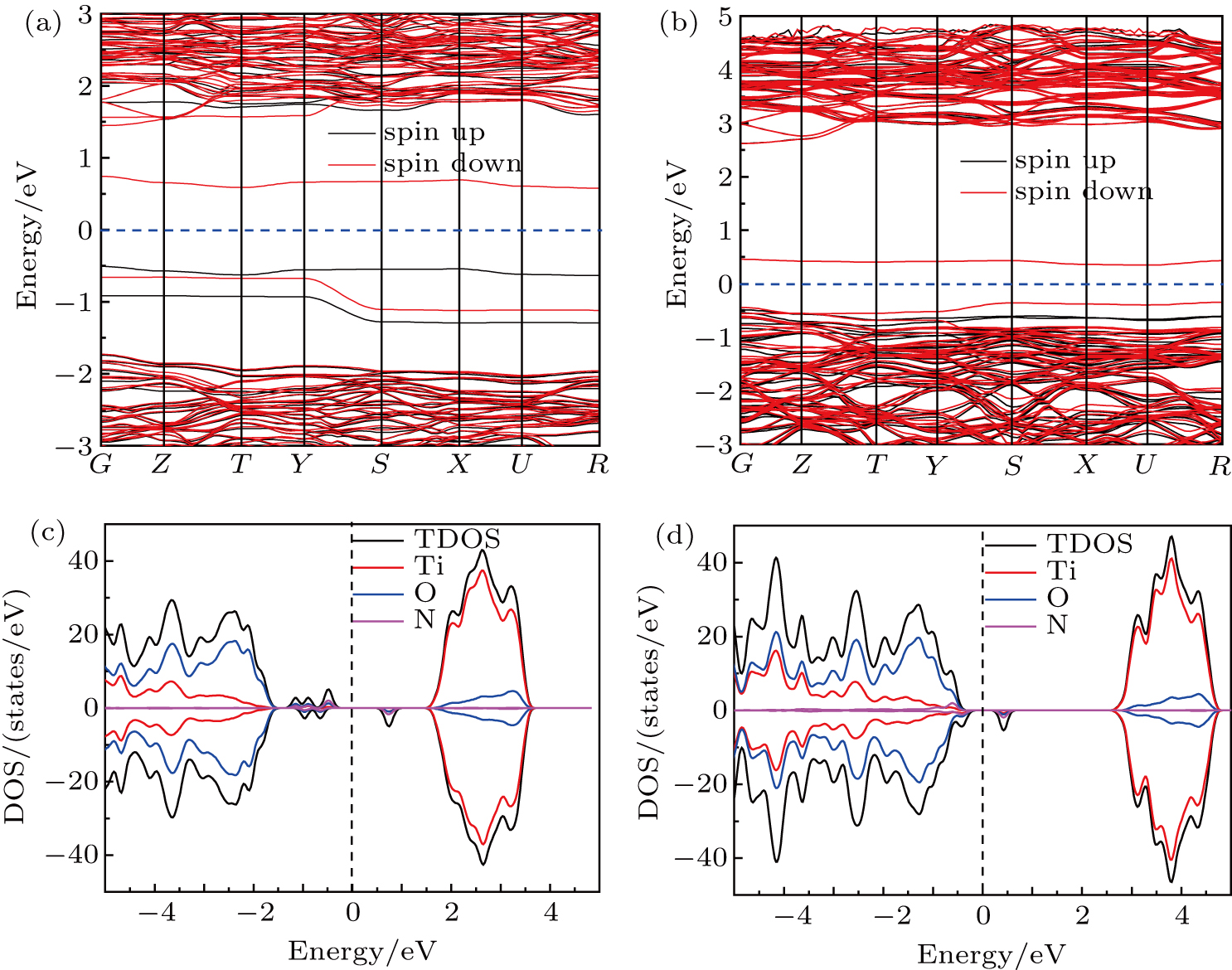 | Fig. 3. The band structure of I–N-doped TiO2 (a), S–N-doped TiO2 (b). The density of states (DOS) of I–N-doped TiO2 (c), S–N doped TiO2 (d). The dashed lines represent the Fermi level EF. |
To analyze the electronic structure of doped TiO2 further, the DOS and projected DOS are shown in Figs.
To better understand the change of photocatalytic activity of nitrogen-doped TNTs, the UV-Vis absorption spectra of the S–N and I–N models are calculated and shown in Fig.
The PC activities of TNTs and nitrogen-doped TNTs have been evaluated under both visible and UV light irradiations. Figures
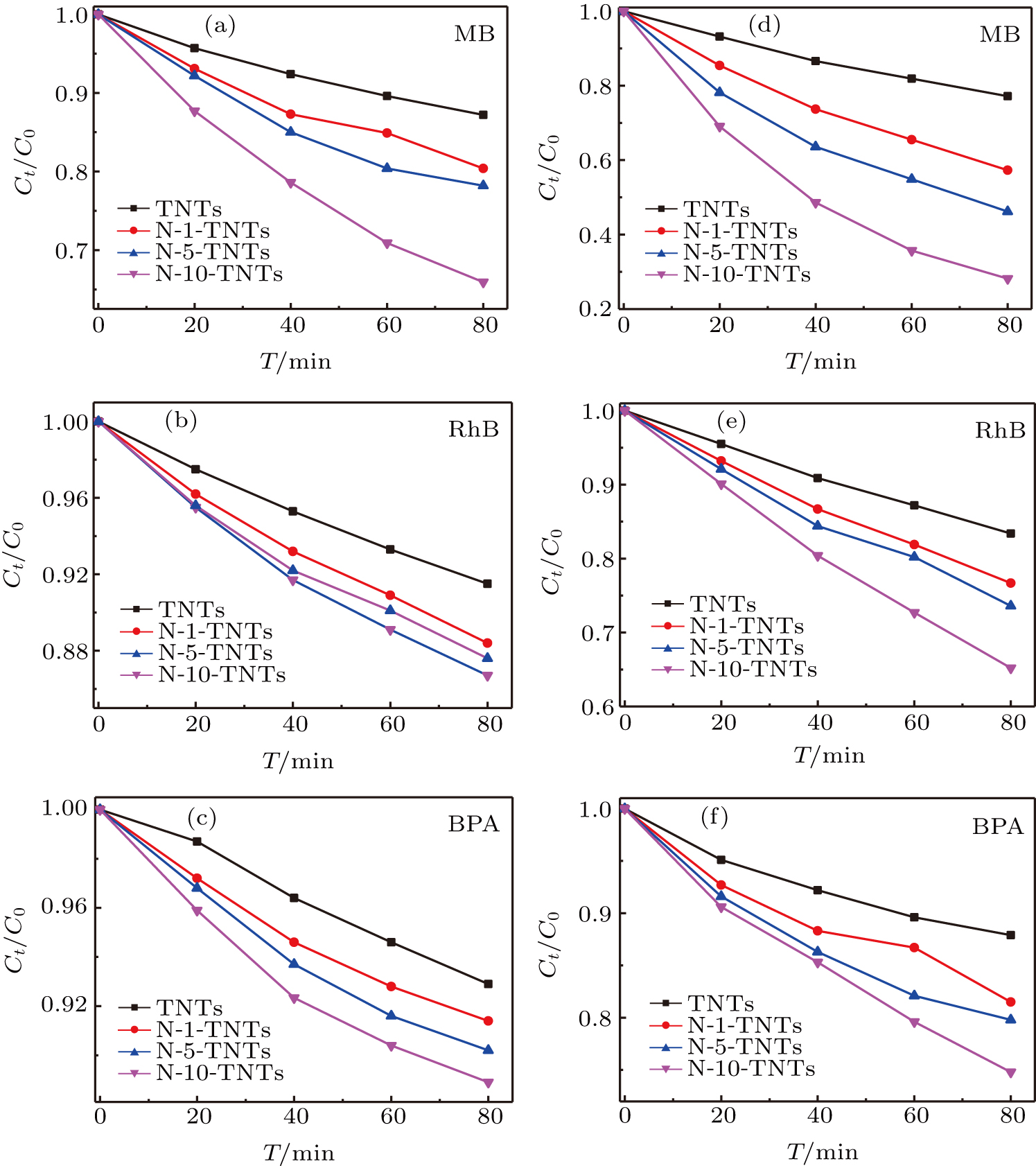 | Fig. 5. Comparison of PC degradation activity for TNTs and nitrogen ions-implanted TNTs under light irradiation: (a)–(c) visible; (d)–(f) UV. |
| Table 1. The PC rate for the degradation of MB, RhB, and BPA on different samples. . |
The k of MB degradation with TNTs under visible light irradiation is only about 0.00179 min−1. However, for the nitrogen ions-doped TNTs, all the k values are enhanced. For example, the k for N-10-TNTs is up to 0.00582 min−1, which is about 3.3 times higher than that of TNTs. In addition, RhB and BPA are degraded by TNTs and nitrogen-doped TNTs to evaluate the activities of the samples further. As shown in Figs.
Figures
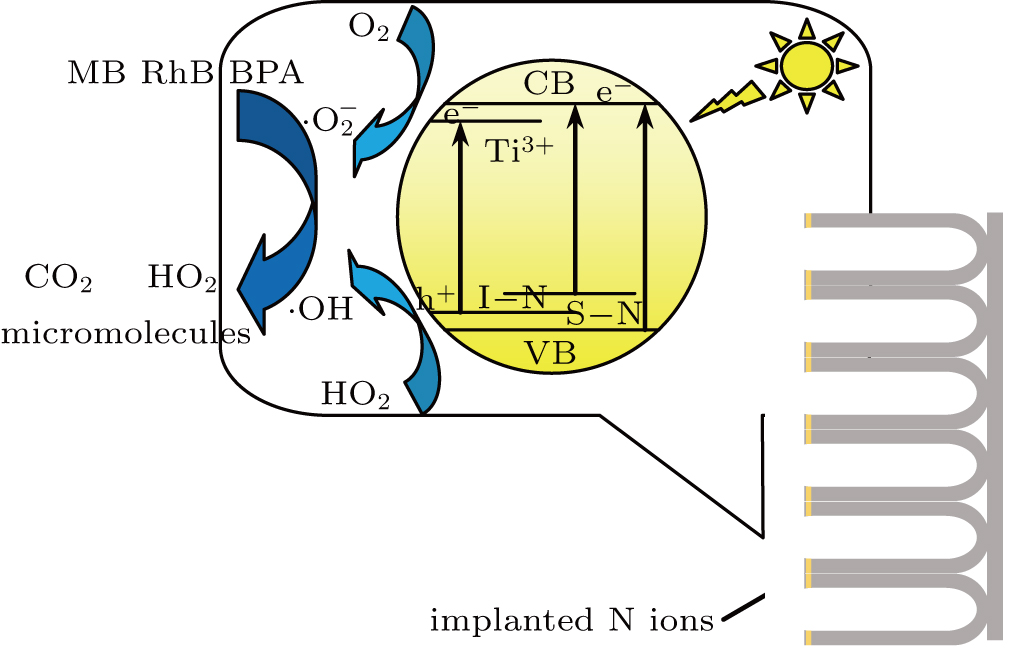
According to the experimental and theoretical results mentioned above, the reasonable mechanism of PC of dyes and chemicals under visible and UV light irradiation is presented in Fig. 
The PC performance of nitrogen-doped TNTs synthesized by ion implantation is improved significantly under both visible and UV light irradiations. The experimental and computational results indicate that impurity energy levels from S–N and I–N dopants are critical for PC performance. The results of PC degradation show that the electrons of CB minimum originate from different excited ways when the implanted samples are irradiated under visible and UV light. The TNTs prepared using ion implantation and anodization show significant visible and UV light-induced PC activity, which could open exciting new opportunities in doping modification of TNTs, especially suitable for doping various non-metal and metal ions into TNTs to improve PC performance.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] |