† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Hebei Province, China (Grant No. E2017203029).
Mn:ZnSe/ZnS/L-Cys core-shell quantum dots (QDs) sensitized La-doped nano-TiO2 thin film (QDSTF) was prepared. X-ray photoelectron spectroscopy (XPS), nanosecond transient photovoltaic (TPV), and steady state surface photovoltaic (SPV) technologies were used for probing the photoelectron behaviors in the Mn-doped QDSTF. The results revealed that the Mn-doped QDSTF had a p-type TPV characteristic. The bottom of the conduction band of the QDs as a sensitizer was just 0.86 eV above that of the La-doped nano-TiO2 thin film, while the acceptor level of the doped Mn2+ ions was located at about 0.39 eV below and near the bottom of the conduction band of the QDs. The intensity of the SPV response of the Mn-doped QDSTF at a specific wavelength was ∼2.1 times higher than that of the undoped QDSTF. The region of the SPV response of the Mn-doped QDSTF was extended by 191 nm to almost the whole visible region as compared with the undoped QDSTF one. And the region of the TPV response of the Mn-doped QDSTF was also obviously wider than that of the undoped QDSTF. These PV characteristics of the Mn-doped QDSTF may be due to the prolonged lifetime and extended diffusion length of photogenerated free charge carriers injected into the sensitized La-doped nano-TiO2 thin film.
Quantum dots (QDs) have been widely studied over two decades[1–9] because of their unique electron structure characteristics such as tunability of the band gap,[10,11] size-dependent extinction coefficient,[12] and multiple exciton effect.[13] As a new material, various QDs are now being used in the fields of fluorescence labeling,[14] photonic crystals,[15,16] photoelectron and microelectron devices,[17] and the third generation solar cells.[18] At present, QDs sensitized solar cells (QDSSCs) have displayed potential applications especially in the renewable energy fields, and a big room for improvement in the photovoltaic (PV) conversion efficiency. Up to now, the study of II/VI group QDSSCs mainly focuses on broadening the visible light absorption range, and increasing the practical conversion efficiency close to the limit conversion efficiency of solar cells sensitized by the QDs.[19–27]
In our previous works, the self-assembled core-shell CdTe/CdS/ligand, CdSe/CdS/ligand, and ZnSe/ZnS/ligand QDs were prepared via the aqueous synthesis way, respectively.[28–31] The results revealed that the ligand and the shell layer played not negligible roles in the gradual energy band structure of these QDs. For examples, the ligand not only improved the stability of the QDs, but also may change the transport mechanism and adjust the transmission channel of photogenerated free charge carriers (FCCs) in these QDs. The outer-layer-ligand of these QDs as sensitizer played a molecular linker role between the QDs and nano-TiO2 substrate, except for those functions mentioned above. The shell layer such as CdS or ZnS in these QDs should be responsible for the obvious quantum confinement effect in these quantum systems, mainly because the energy band of the shell layer was located in between those of the core and the outer layer ligand. The study confirmed that ZnSe QDs-sensitized nano-TiO2 thin film (QDSTF) may become a potential candidate of photoanode, and the photoanodic properties probably determined the overall performance of QDSSCs, because the ZnSe QDs sensitizer has better surface photovoltaic (SPV) characteristics and lower toxicity than other members of II/VI semiconductors.[32] Moreover, some characteristic of the photoanode was closely related to the extended diffusion length of photogenerated FCCs and increased level of electron injection into the nano-TiO2 substrate from the QDs sensitizer.[33]
It was reported that doped QDs can improve some performances of QDSSC.[34] In order to explore the effect of doping manganese in ZnSe QDs on the photoelectron characteristics of the QDSTF, in the present paper, Mn-doped ZnSe QDs were synthesized by a low temperature aqueous-phase method, and then Mn:ZnSe QDSTF was prepared by a modified chemical bath deposition (CBD) method. The assisting role of the La-doped mesoporous nano-TiO2 substrate, for improving the carrier transport environment of the Mn:ZnSe QDSTF, was discussed in the paper. A series of photophysical phenomena of the Mn-doped QDSTF were probed via nanosecond transient photovoltaic (TPV) and steady state SPV technologies, x-ray photoelectron spectroscopy (XPS), and UV-VIS absorption spectrum, supplemented by Fourier transform infrared (FTIR) spectrum, scanning electron microscopy (SEM), and laser Raman spectrum.
First, La-doped mesoporous nano-TiO2 powder was synthesized by the sol–gel method, in which polyethylene glycol was used as a template.[35,36] Figure
The modified aqueous synthesis of Mn-doped ZnSe QDs capped by L-Cys, similar to Ref. [29], involved the reaction between selenium-, zinc-, and manganese-precursors. First, the selenium precursor was prepared by mixing selenium powder with a NaBH4 solution under stirring until the reaction was completed. The achieved selenium precursor with the concentration of sodium hydrogen selenide (NaHSe) equal to 0.167 mol/L was saved under the protection of nitrogen. Then, Zn(CH3COO)2, L-Cys, and Mn(CH3COO)2 were dissolved into deionized water with stirring and degassing with N2 at pH 11 for 30 min. And then, the as-prepared Se-precursor was added to the above mixed solution. The Mn-doped ZnSe QDs solution was obtained after the mixed solution of selenium-, zinc- and manganese-precursors reacted at the room temperature in the atmosphere of nitrogen for 3 h.
Mn-doped ZnSe QDs sensitized La-doped nano-TiO2 thin film was prepared by the CBD method.[38] Briefly, the TiO2 thin film was placed at a certain angle in a beaker containing the Mn-doped ZnSe QDs solution, and then transferred with the beaker to an oil bath, deposited at 60 °C for 5 h. The sensitized thin film was taken out and rinsed with deionized water several times. Finally, the QDs sensitized thin film was obtained after it was dried at 60 °C for 0.5 h.
XRD (Rigaku D/max-2500/PC, Japan) was used to study the crystal structure of the as-prepared samples. The Brunauer–Emmett–Teller (BET) method (ASAP2020 HD88, USA) was used for detecting the specific surface area and porous structure of the as-prepared samples. XPS (Thermo ESCALAB 250 spectrometer with Al Kα excitation 1486 eV, USA) was used to provide the information about the composition and valence state of the elements in the as-prepared QDs. SEM (Hitachi S-4800, Japan) was used to study the micromorphology of the as-prepared samples. Raman spectroscopy (Renishaw, England) was employed to inspect the molecular structure in the as-prepared nano-TiO2 thin films.
In our experiment, SPV and TPV spectra of the as-prepared samples were detected by self-assembling devices, respectively. Details for the SPV and TPV measurements were described elsewhere.[39,40] SPV and TPV techniques can be used to obtain information on photoelectron behaviors at surfaces and phase interfaces because these techniques are by no means only sensitive to surfaces. Instead, they are sensitive to the entire surface space charge regions (SCRs) by super-or sub-band gap absorption, and even to buried interfaces located anywhere in the detected sample, as long as they can be reached by photons.[41]
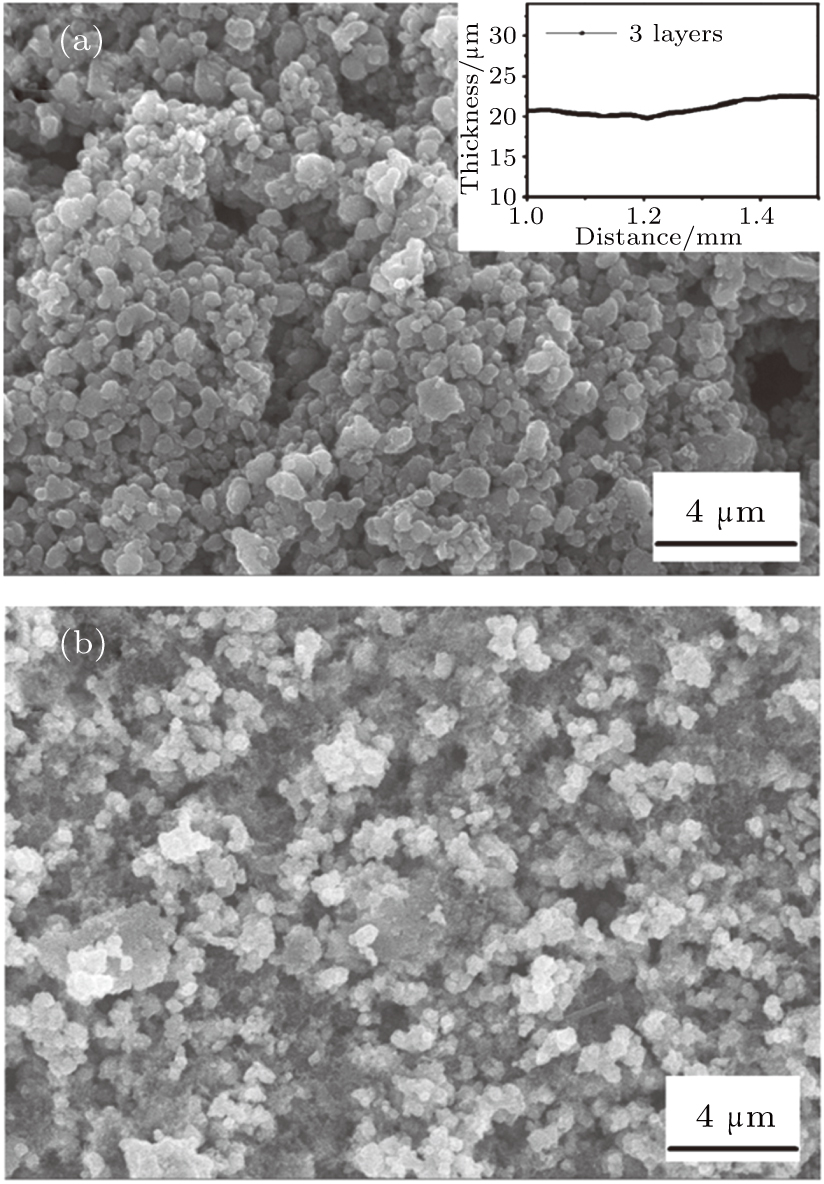
Figure
Figure
Laser Raman spectra of the thin films before and after sensitized by the Mn-doped QDs are shown in Fig.
 | Fig. 3. Raman spectra of the nano-TiO2 thin film before and after sensitized by the Mn-doped ZnSe QDs. Inset is the Raman spectrum of crystalline ZnSe. Laser: 532 nm edge (mode: regular). |
FTIR spectra of the as-prepared La-doped nano-TiO2 thin film, undoped QDSTF, Mn-doped QDSTF, and pure L-Cys ligand are shown in Fig.
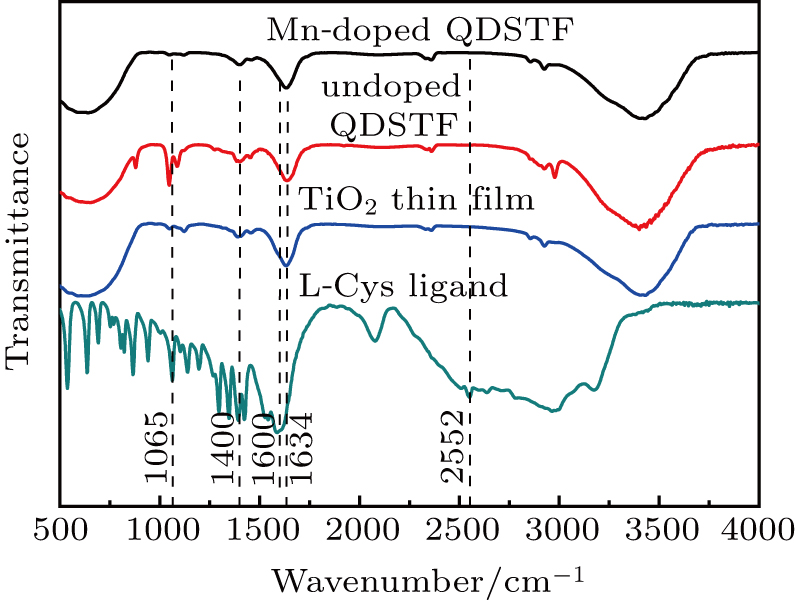 | Fig. 4. FTIR spectra of the pure ligand L-Cys, the La-doped nano-TiO2 thin film, the undoped QDSTF, and the Mn-doped QDSTF. |
| Table 1. Wavenumbers (cm−1) corresponding to some chemical bond vibrations extracted from the FTIR spectra of the four samples. . |
Room temperature UV-VIS absorption spectra of three types of nano-TiO2 thin films are shown in Figs.
| Table 2. Relevant parameters extracted from the UV-VIS absorption spectrum, SPV spectroscopy, and TPV spectroscopy of the as-prepared undoped ZnSe QDs-sensitized nano-TiO2 thin film and the as-prepared Mn:ZnSe QDs-sensitized nano-TiO2 thin film. . |
Figure
According to Ref. [60], the time range of the TPV response depends on the proportion of the thickness of SCR to the length of the photogenerated FCCs drift in SCR, and/or on the diffusion distance of the electron–hole pairs. Therefore, the diffusion length of the photogenerated electron-hole pairs in the Mn-doped QDSTF should be much larger than that in the undoped QDSTF, because the start time of the TPV response was ahead from 8.36 × 10−8 s to 7.53 × 10−8 s, and the end time was prolonged from 2.70 × 10−3 s to 6.10 × 10−3 s as compared the former to the latter, as shown in Fig.
SPV spectroscopy of La-doped TiO2 nanoparticles and undoped TiO2 nanoparticles is displayed in Fig.
In summary, the combination of the low temperature aqueous-phase way and the modified CBD method was used to prepare Mn:ZnSe/ZnS/L-Cys core-shell QDs sensitized La-doped nano-TiO2 thin film. Photoelectron characteristics and microstructure of the Mn-doped QDSTF were probed via XPS and transient and steady state PV technologies, supplemented by FTIR spectrum, UV-VIS absorption spectrum, and laser Raman spectrum. The results confirmed that Mn element was doped into the ZnSe QDs in Mn2+ ion form, which partly replaced the vacancy of Zn2+ ion and formed MnSe with Se2− ion at the low temperature. The ligand L-Cys not only was a stabilizer of the Mn-doped ZnSe QDs sensitizer, but also played a role of molecular linker between the sensitizer and the substrate. The research revealed that the La-doped nano-TiO2 thin film provided bigger specific surface and porosity, and was composed of anatase phase with better photoelectric property rather than rutile phase, as compared with the undoped nano-TiO2 thin film. The results verified that the bottom of the conduction band of the Mn:ZnSe QDs as a sensitizer was just 0.86 eV above that of the substrate thin film. And the acceptor level of the doped Mn2+ ion was located at about 0.39 eV below and near the bottom of the conduction band of the QDs. The study confirmed that the Mn-doped QDSTF had a p-type TPV characteristic unlike the undoped QDSTF. The advantages of the Mn-doped QDSTF were strongly dependent on the microstructures mentioned above. Specifically, the intensity of the SPV response of the Mn-doped QDSTF at the specific wavelength was approx. 2.1 times higher than that of the undoped QDSTF. The region of the SPV response of the Mn-doped QDSTF was extended by 191 nm to almost the whole visible region as compared with that of the undoped QDSTF. The region of the TPV response of the Mn-doped QDSTF also was obviously wider than that of the undoped QDSTF although the extreme of the TPV response of the former was lower than the latter. These outstanding PV characteristics of the Mn-doped QDSTF probably were derived from the prolonged lifetime and extended diffusion length of photogenerated FCCs injected into the La-doped nano-TiO2 substrate from the Mn:ZnSe QDs sensitizer.
The adsorption–desorption isotherm and the pore size distribution pattern of the as-prepared TiO2 nanoparticles are shown in Fig.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] |