Advanced characterization and calculation methods for rechargeable battery materials in multiple scales
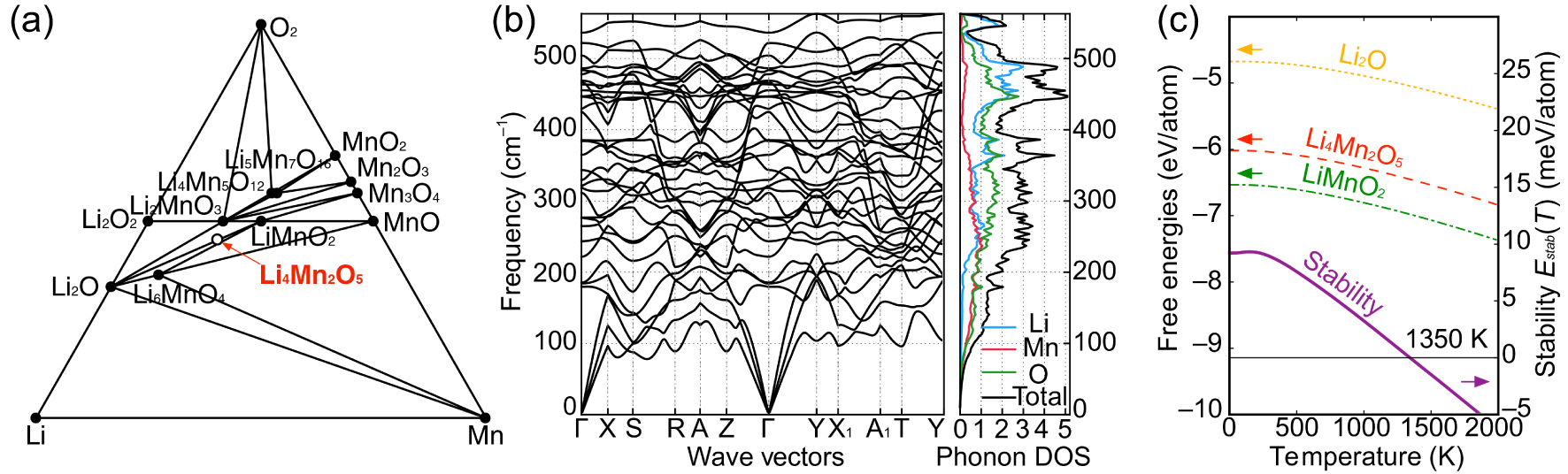
Thermodynamic and dynamic stabilities of Li4Mn2O5. (a) Calculated Li–Mn–O (T = 0 K) phase diagram. The Li4Mn2O5 phase is slightly higher in energy (13.6 meV per atom) relative to the ground-state phase: a mixture of Li2O and LiMnO2. (b) Phonon dispersion of the ground-state Li4Mn2O5 and (c) calculated temperature-dependent free energy of Li4Mn2O5 (red dashed line), Li2O (orange dotted line), and LiMnO2 (green dotted line), as well as the stability (purple solid line) of Li4Mn2O5 versus temperature relative to Li2O and LiMnO2 phase mixtures. Li4Mn2O5 is dynamically stable and can be entropically stabilized at ∼ 1350 K.[