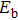† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11574167 and 11874033) and the KC Wong Magna Foundation in Ningbo University, China.
It is critical to design an effective two-dimensional membrane for hydrogen purification from the mixed gas, due to its wide range of scientific and industrial applications. In this work, we investigate the hydrogen separation performance of P2C3 membranes by density functional theory and molecular dynamics simulations. The results show that the energy barrier of the H2 molecule through the P2C3 film is only 0.18 eV, while the energy barriers of the CO, N2, CO2, and CH4 molecules are 0.77 eV, 0.87 eV, 0.52 eV, and 1.75 eV, respectively. In addition, the P2C3 film has high H2 selectivity toward other gas molecules and high H2 permeability at room temperature. Under 6% tensile strain, 82% hydrogen molecules pass through the film with a H2 permeance of 2.22 × 107 gas permeance unit (GPU), while other molecules cannot across the membrane at all. Therefore, the P2C3 membrane is an excellent material for hydrogen purification.
It is great important to efficiently separate the light gases (H2, N2, CO2, CO, and CH4) because most of them are frequently used raw materials in energy, scientific, and chemical fields.[1,2] Under ambient condition, hydrogen is a highly flammable, colorless, transparent, odorless, tasteless, and insoluble gas. Conventional gas separation techniques are complicated to operate and consume a lot of energy, such as cryogenic distillation, pressure swing adsorption polymers, zeolites, and metal–organic frameworks.[3,4] Membrane separation with atomic thickness has emerged, with many advantages, such as suitable pore size to solve permeability and selectivity, high efficiency, ease of operation, and environmental friendliness, soon it has became an ideal candidate for gas separation and caused great interest.[5–10] Since the successful synthesis of graphene in 2004,[11] porous graphene nano-sheets have been widely reported for gas separation due to their extraordinary thermodynamic stability and dynamic stability.[11–13] Jiang et al., using the first-principles density functional theory (DFT) calculations, showed that the designed sub-nanometer porous graphene sheets have high H2 transmission and high H2/CH4 selectivity (about 108). Koenig used ultraviolet-induced oxidative etching to produce micrometer-sized graphene films, which could be used as molecular sieves.[14] The experimental results effectively validated the theoretical prediction of porous graphene with gas separation ability. In recent years, to enhance the permeability and selectivity for H2 and He purification, novel two-dimensional (2D) nanomaterials with various pore sizes have been successively applied to gas separation.[16–20] Still, it is highly desired to develop new 2D membranes with inherently ideal pore sizes for mixed gas separation.
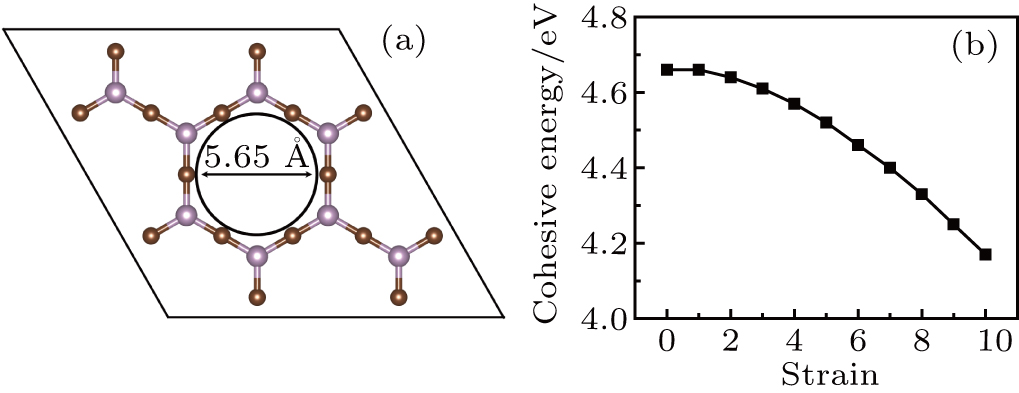
Recently, Chen et al.[21] proposed a micrometer-sized monolayer of phosphorus carbide P2C3, a porous structure containing P. The pores of this membrane are uniformly distributed, with a diameter of 5.65 Å, which is much larger than the kinetic diameter of H2 molecule (2.89 Å). Importantly, ab initio molecular dynamics (AIMD) simulations showed that, even at temperature up to 1100 K, the P2C3 sheet retains its structural integrity and has no soft mode, indicating remarkable thermal stability. It can be expected that the P2C3 film could exhibit good H2 separation performance.
In this work, we investigate the H2 separation performance of the P2C3 monolayer based on the DFT and AIMD simulations. The results show that the stability of the porous P2C3 monolayer is sufficiently high to be used as a separation membrane. The energy barrier for the considered gas molecules passing through P2C3 membrane is clearly divided into two camps, the barrier of H2 is lower than 0.2 eV, while the values of CO, N2, CO2, and CH4 are all above 0.5 eV. To further estimate the H2 separation performance of the P2C3 sheet, the gas selectivity and H2 permeance are analyzed.
Our calculations were carried out using the Vienna ab initio simulation package (VASP).[22,23] The exchange–correlation interactions were described by a generalized gradient approximation (GGA)[24] with the Perdew–Burke–Ernzerhof (PBE) functional.[25] A damped van der Waals correction in Grimme’s scheme (DFT + D2)[26] was included in all the calculations. The cut-off energy for plane waves was set at 500 eV, and the convergence criteria for force and energy on each atom during structure relaxation were set to 0.005 eV·Å−1 and 10−5 eV, respectively. To avoid the interaction between the neighboring periodic structures, we adopted periodic boundary conditions with a vacuum space of 20 Å. The climbing image nudged elastic band (CINEB) was used to search the transition state (TS) when gas diffused through the pores. The 2 × 2 unit cell of P2C3 was used as the model for CINEB calculations. The Brillouin zone was sampled with the Monkhorst–Pack k-mesh of 5 × 5 × 1 in reciprocal space during the geometry optimization and dynamics. MD simulations were performed in the canonical ensemble (NVT) using Nosé–Hoover thermostat to control the temperature.[27] The condensed-phase optimized molecular potential for atomistic simulation studies (COMPASS) force-field was used to performance the atomic interactions. The atom-based van der Waals interactions and Ewald electrostatic interaction were set with a cutoff distance of 12.5 Å. Every simulation ran for 5 ns with a time step of 1.0 fs.
The optimized geometry of P2C3 is shown in Fig.
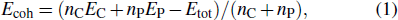 |
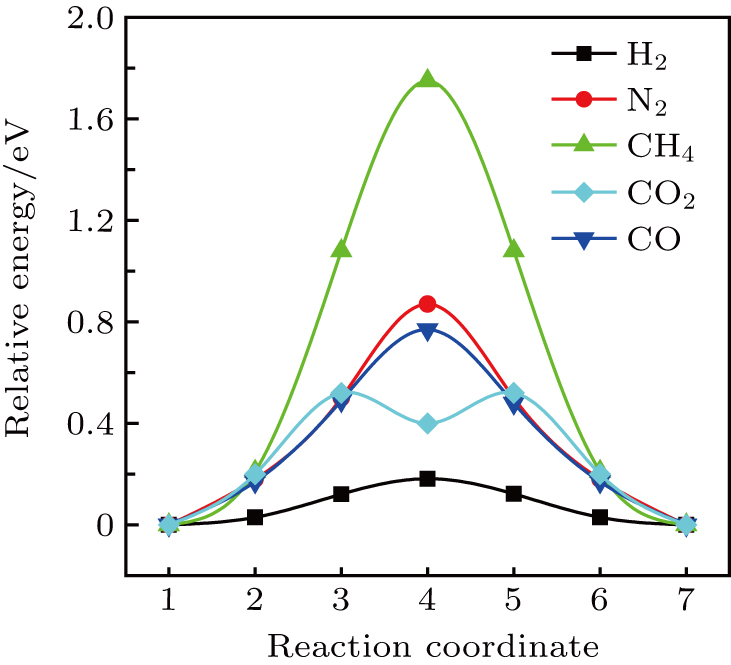
During the experimental process, strain is unavoidable. Tensile strain would increase the pore size and reduce the cohesive energy. In Fig.
Using the CINEB method, we calculate the energy barrier of the gas molecules passing through the pores, which is defined as
 |
In Table
 | Fig. 3. Electron density isosurfaces when (a) H2 and (b) CH4 molecules passing through P2C3 membrane. The isovalue is set to be 0.015 e/Å3. |
| Table 1 The kinetic diameter D and the energy barrier |
With the energy barrier Eb, we use the Arrhenius equation[32] to calculate the selectivity of H2 relative to other gas molecules, which can be given as
 |
 | Fig. 4. (a) Diffusion rates of all the studied gas molecules (H2, CO, CO2, N2, CH4) and (b) H2 selectivity relative to other gas molecules as a function of temperature. |
Permeability is another important criterion for evaluating the H2 separation performance of the P2C3 sheet. We investigate whether mixed gas molecules (composed of 72 H2, 36 CO, 36 CO2, 36 CH4, 36 N2 molecules) at room temperature can pass through the film. The permeability can be obtained by
 |
 | Fig. 5. The snapshot of MD simulations for the gas mixture permeating through (a) the pristine P2C3 membrane and (b) the 8% stretched membrane at 300 K. |
The penetration rate of hydrogen gas can be further increased by increasing the size of the molecular sieve by mechanical strain. We consider the different tensile strains and find that applying 6% strain to the membrane not only improves the H2 pass rate (the penetration of H2 can reach 2.22 × 107 GPU), but also successfully prevents other molecules from passing through. Under the 8% strain, the carbon dioxide molecules begin to pass through the membrane (see Fig.
Based on DFT and MD simulations, we investigate the diffusion and selectivity of hydrogen, carbon monoxide, nitrogen, carbon dioxide, and methane through a porous P2C3 film. The results show that the energy barrier of hydrogen passing through the membrane is as low as 0.18 eV, while the values of other molecules are higher than 0.5 eV, which indicates that hydrogen and other gases can be effectively separated in one step. At room temperature, the P2C3 membrane has a very high H2 permeability, when the mechanical strain increases to 6%, the permeability can reach 2.22 × 107 GPU. We predict that the P2C3 membrane is an excellent material for hydrogen purification in terms of H2 selectivity and permeability.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] |