Ultra-high-density local structure in liquid water
Project supported by the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (Grant No. QYZDB-SSW-SYS003) and the National Natural Science Foundation of China (Grant Nos. 11574310 and 11774394).
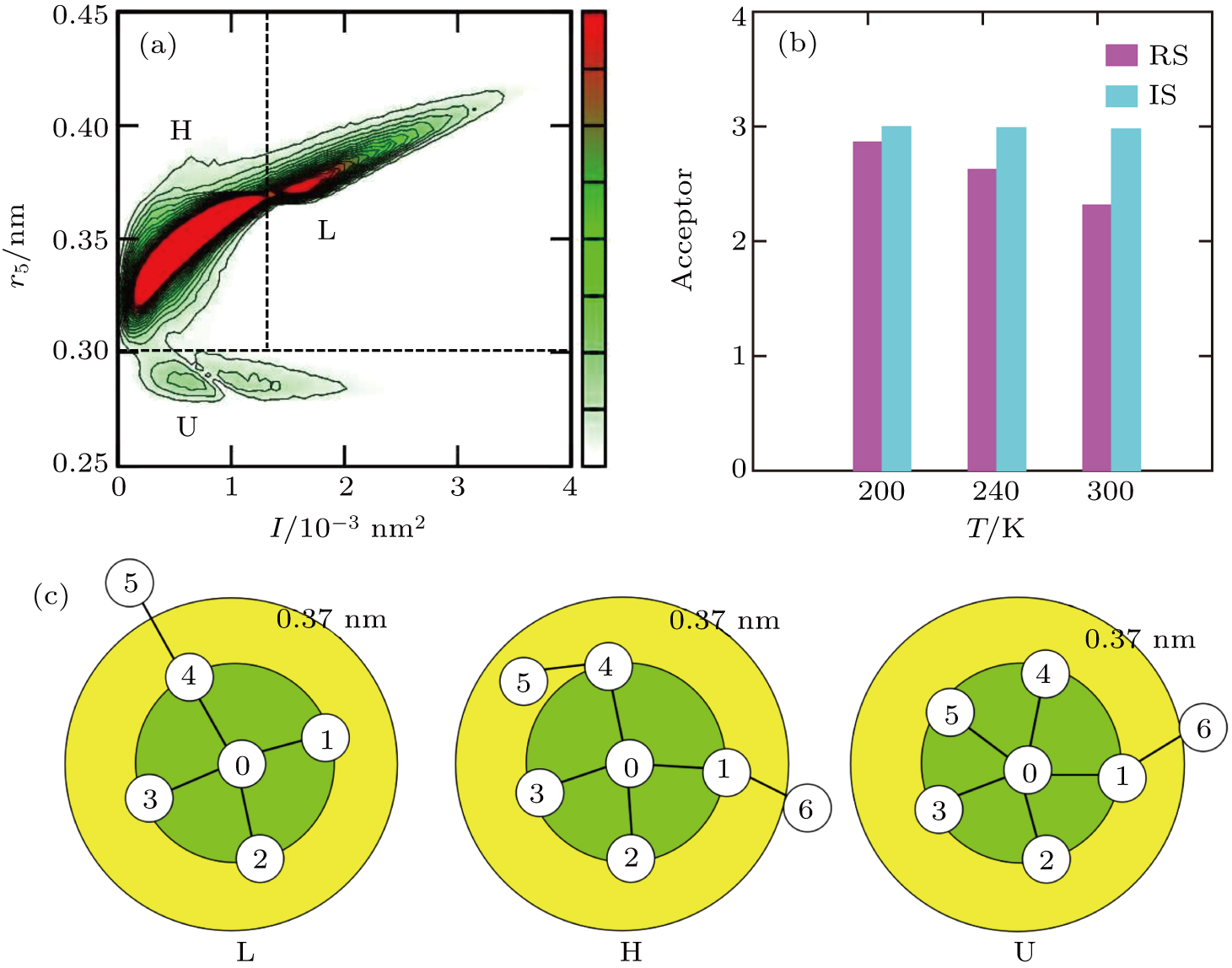
Identification and characteristics of the third type water molecules. (a) Joint I–r5 distribution at 1 atm and 300 K in the inherent structure, where the values of the probability density are given by the color bar on the right. The three peaks corresponding to LDL, HDL, and UHDL have been labeled with L, H, and U, respectively. The boundary between the UHDL and HDL is r5 = 0.305 nm and the boundary between the HDL and LDL is I = 1.35 × 10−3 nm2. (b) Average number of acceptor bonds of UHDL at various temperatures. In the inherent structure, the acceptor bond number is always close to three at all temperatures; in the real structure, due to thermal fluctuation, the acceptor bond number decreases when the temperature increases, but still remains bigger than two even at 300 K. (c) Schematic illustration of three species in the inherent structure, where the fifth neighbor of the LDL molecule locates beyond the 0.37 nm circle and that of the HDL molecule is in the gap between the first and second shells, while the UHDL molecule has five neighbors in the first shell.