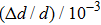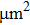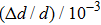† Corresponding author. E-mail:
We study the absorption of hydrogen of metal by the permeability method. With the help of the gas reaction controller (GRC), the absorptive capacity of hydrogen, which is a function of time, temperature and pressure, can be recorded. The effect of the performance of the hydrogen permeability of AlN coating on the titanium alloy surface structure is studied. In the research, the AlN is selected to be added to the titanium alloy sample VT6, and the properties of the titanium alloy are investigated, and the hydrogen absorption rate of the coating is calculated by performing the hydrogen saturation of the test sample. The results show that under 600 °C the AlN film reduces the hydrogen absorption rate of titanium alloy and improves the surface properties of VT6 alloy.
Due to their high strength, low specific gravity, high corrosion resistance, and high biological capacity, titanium alloys are widely used in medical, automotive, aerospace, and chemical industries.[1,2] One of the main problems in using the titanium alloy products originates from hydrogen.[3] The hydrogen dissolved in titanium alloys can lead to significant changes in physical and mechanical properties of the alloys. It is almost impossible to prevent hydrogen from entering into materials because of the high content of hydrogen in the environment and the limitation of technical conditions. Although the existence of hydrogen embrittlement has been discovered for a long time, it is still worth studding how to protect titanium alloy from producing the hydrogen embrittlement.
In order to avoid producing the hydrogen embrittlement, the hydrogen content in alloy must be limited. Vacuum annealing is necessary when hydrogen exceeds a maximum allowable value in titanium and its alloys.[3] But it is a very time-consuming and expensive process. Therefore, one tends to choose alloys that are more tolerant to hydrogen penetration, or are not easy to embrittle under special circumstances. To reduce the negative effects of hydrogen, it is useful to reduce the ambient gas pressure to a low level, at which no hydrogen can permeate. But this method brings the complexity to the structure. So it is difficult to further improve the aviation and rocket technology.
The chemical properties of titanium are very active because the ionization energy of the outer electrons of titanium is very small and the outer electrons are easy to lose. Titanium is easily oxidized to form oxide film. This oxide film is continuous and very compact, and is stable in most of environments. The passive film can effectively prevent gas from being penetrated. However, at high temperatures, the oxide film will decompose, and lead hydrogen permeability to increase significantly.
The methods of dealing with hydrogen vulnerability are as follows: (i) adding coatings to reduce hydrogen permeation and accumulation; (ii) improving alloy properties, reducing the interaction rate of metals with hydrogen and increasing maximum allowable hydrogen concentration; (iii) heat treatment of products.[4] The ALN coating is chosen in this work. Aluminum nitride can work in hydrogen and carbon dioxide atmospheres at 980 °C. It has good structural stability at high temperature and good thermal conductivity.
The hydrogen saturation of the sample is studied by the automatic gas reaction controller. The automatic gas reaction controller is a system for studying the absorption properties of hydrogen on materials, in which the hydrogen saturation of samples comes from the gas environment.
The control software is programmed based on LabVIEW. The high temperature furnace consists of constant temperature furnace, low pressure furnace, high pressure furnace, chamber and controller. The controller consists of an electronic control system and a vacuum system. The vacuum part includes volume control system, low pressure tank, high pressure tank, pneumatic control valve, manual valve, pressure gauge and filter. An emergency hydrogen removal system is installed to release hydrogen when the maximum allowable pressure is exceeded to ensure the safety between tanks. A vacuum unit consisting of a diaphragm pump and a turbomolecular pump is used to generate vacuum in a vacuum system. Hydrogen is supplied from the cylinder or hydrogen generator. To prevent the connection from being damaged by heat, a cooling system is installed between the furnace and the controller.
The principle of operation of the experimental setup is described as follows (see Fig.
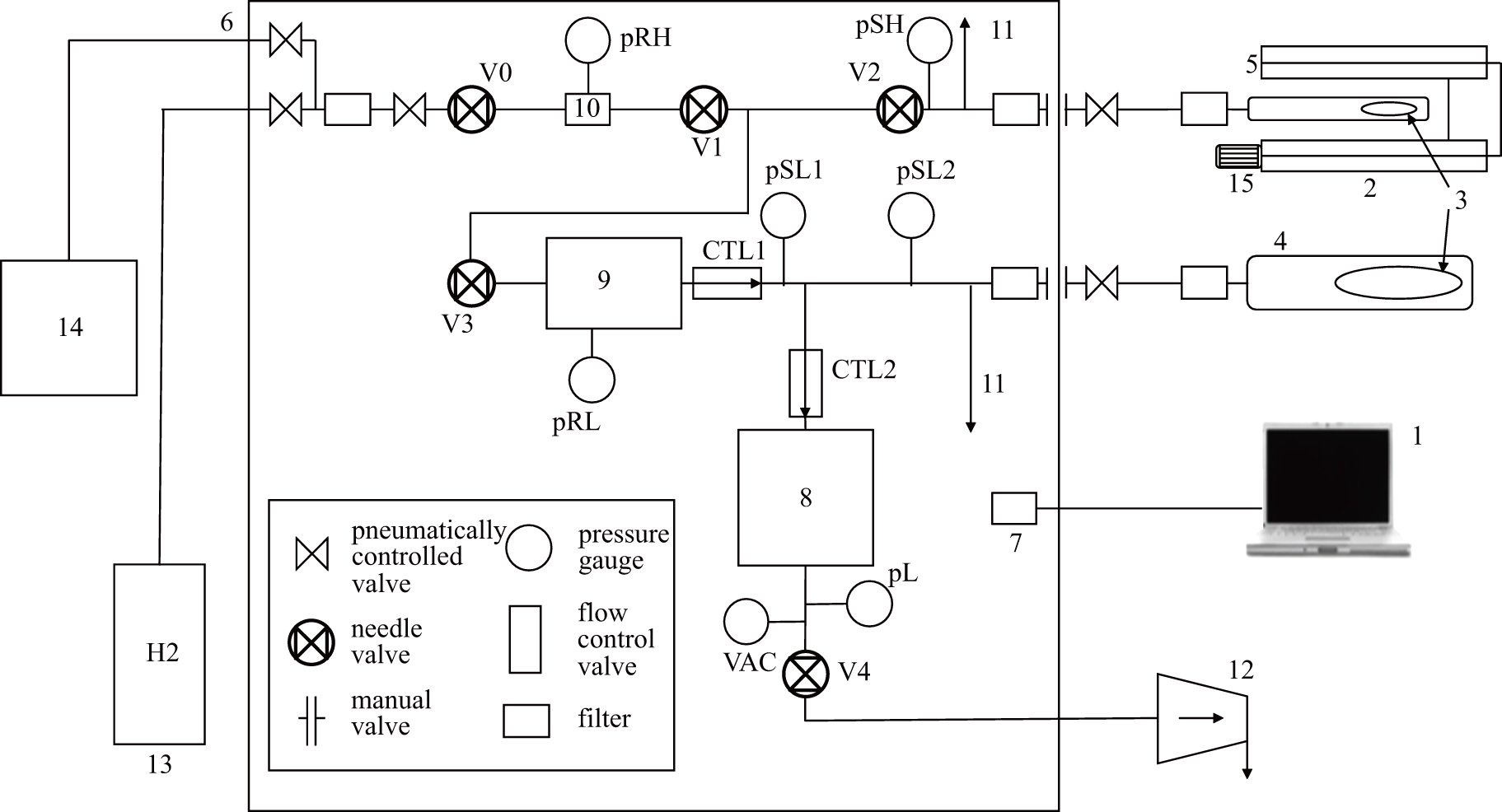 | Fig. 1. Gas reaction controller: 1: computer; 2: constant temperature furnace; 3: sample; 4: low-pressure chamber; 5: high-pressure chamber; 6: controller; 7: electronic control system; 8: control volume; 9: low-pressure chamber; 10: high-pressure chamber; 11: emergency hydrogen removal system; 12: vacuum component; 13: hydrogen tank; 14: hydrogen generator; 15: cooling systems.[5] |
VT6 titanium alloy is used as a research material. The sample is a 20 mm×20 mm ×1 mm rectangular sheet, and is polished to 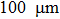
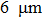
After hydrogen saturation, we measure the hydrogen concentration with an RHEN 602 (LECO) hydrogen analyzer. The elemental analysis of the samples is carried out by GD Profiler 2 (Horiba) glow discharge plasma spectrometer, and the structural phase analysis is carried out by XRD analyzer with using Cu Kα radiation. The XRD analyze is measured at angles ranging from 30° to 80° and at a speed of 10°/min. PCPDFWIN, PDF4+ database and POWDERCELL 2.4 analysis program are used for phase composition analysis. The surface structure of the coated samples is observed by scanning electron microscopy. Hardness is measured by the Vickers method. The Vickers diamond hardness is a function of test load (F) divided by imprinted surface area (1.8544/d2). The Vickers diamond hardness is calculated from the following formula:
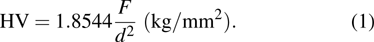 |
Figure 
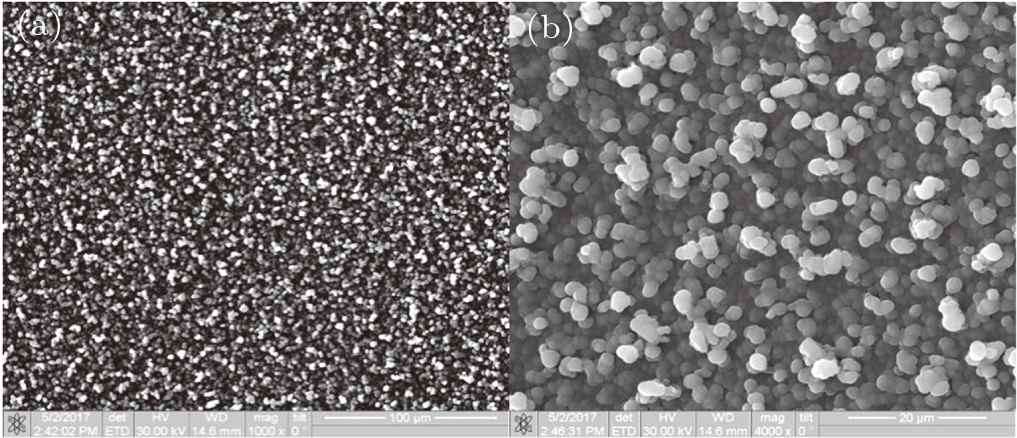 | Fig. 2. Surface structure of VT6 alloy after ALN coating with magnification (a) 1000x, and (b) 4000x. |
| Table 1.
Microanalysis of energy dispersion of coated VT6 alloy. . |
| Table 2.
Results of XRD analysis of sample structure. . |
The mechanical properties of the samples are evaluated by measuring microhardness and wear resistance.
The results of the study of the wear resistance of VT6 alloy before and after AlN coating are shown in Table 
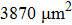
| Table 3.
Wear resistances of samples. . |
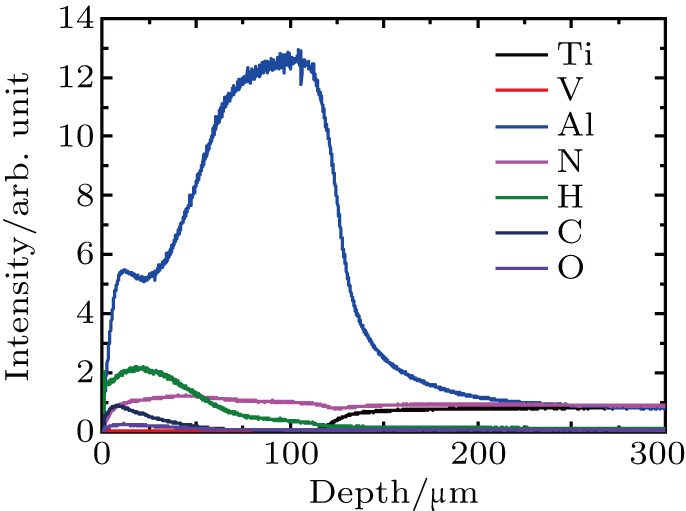
High frequency glow discharge optical emission spectrometry is used to evaluate the element distribution and coating thickness of the experimental sample. Figure
In addition to the basic elements of AlN coatings and titanium alloy VT6 hydrogen content is observed to increase on the surface after coating (Fig.
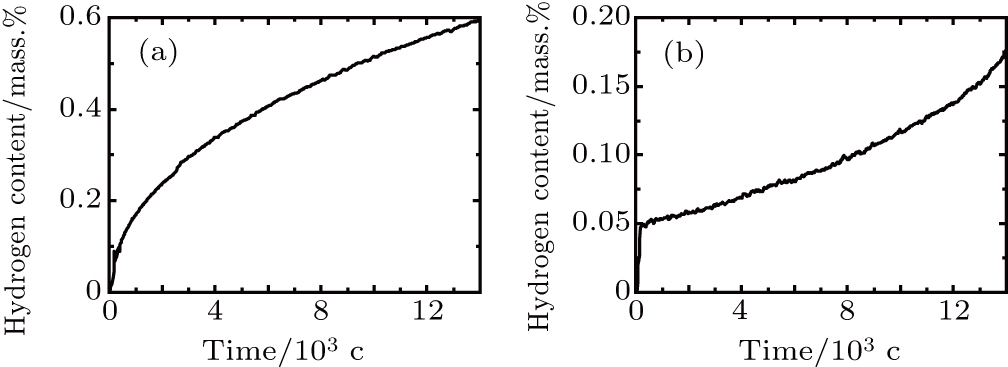
Figure
It can be observed that the hydrogen absorption rate of VT6 alloy decreases due to AlN coating. After coating, the hydrogen absorption rates of the raw materials are 4.3×10−5 mass%/min and 1.5×10−5 mass%/min, respectively. Hydrogen absorption of titanium depends on many factors. The hydrogen absorption rate of titanium increases with the decrease of the number of micro-particles. The oxygen and nitrogen in titanium slow down the rate of hydrogen absorption by titanium. In particular, the absorption rate of hydrogen by oxide film on titanium surface decreases significantly.
The results of XRD analysis are listed in Table
| Table 4.
XRD analysis of hydrogen saturated samples. . |
| Table 5.
Hardness values of VT6 alloy before and after coating. . |
It can be ensured that the application of the AIN coating results in the microhardness of the surface of the titanium alloy increasing 1.7 times. The increase of hardness of coated sample is related to aluminum nitride, and the hardness is significantly higher than the hardness of titanium. At the same time, the hardness of the hydrogenated sample is about 40% lower than that of the coated unsaturated sample. As fragile hydride phases are formed in titanium alloys, the hardness decreases after hydrogenation, which is confirmed by the results of x-ray analysis (Table
Dense AlN thin films are obtained by vacuum arc ion plating. The interaction between hydrogen and AlN coating is studied. It can be confirmed that the wear resistance of VT6 alloy can be increased twice by the AlN coating, and the surface microhardness of titanium alloy can be increased by 1.7 times. The increase of hardness of coated samples is related to aluminum nitride, and the hardness is significantly higher than the hardness of titanium. The hydrogen absorption rate of VT6 alloy decreases due to AlN coating. The hardness of the hydrogenated sample is about 40% lower than that of the coated unsaturated sample. After AlN coating, the permeation rate of hydrogen to titanium alloy decreases obviously.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 |