† Corresponding author. E-mail:
Electrides are unique ionic compounds that electrons serve as the anions. Many electrides with fascinating physical and chemical properties have been discovered at ambient condition. Under pressure, electrides are also revealed to be ubiquitous crystal morphology, enriching the geometrical topologies and electronic properties of electrides. In this Review, we overview the formation mechanism of high-pressure electrides (HPEs) and outline a scheme for exploring new HPEs from pre-design, CALYPSO assisted structural searches, indicators for electrides, to experimental synthesis. Moreover, the evolution of electronic dimensionality under compression is also discussed to better understand the dimensional distribution of anionic electrons in HPEs.
As an emerging class of unconventional ionic solids, electrides have attracted considerable attention in recent years. In electrides, the excess electrons are confined in the vacant crystallographic sites of the lattices, serving as anions.[1–3] These anionic electrons are originated from cations and independent of any particular atom or molecule in the host structures. Due to the loose binding nature of anionic electrons, electrides exhibit many interesting physical and chemical properties, such as high electronic mobility,[4–6] ultra-low work functions,[7,8] and anisotropic electronic and optical responses,[9,10] which make electrides the promising materials in high performance catalysts,[11–14] organic light-emitting diodes (OLEDs),[15,16] superconductors[17–19] and novel electrode materials for batteries.[20,21] The first crystalline electride of Cs+(18C6)2e− was found in organic compounds by Dye et al. in 1983,[22] which are constituted by alkali elements and organic complexant. Afterwards, several organic electrides have been discovered, e.g., K+(cryptand-2.2.2)e−,[23] [Cs+(15C5)(18C6)e−]6(18C6),[24] and Li+(cryptand-2.1.1)e−.[25] However, the very poor thermal stability, extreme air- and water-sensitivity of organic electrides inhibits their potential applications. In 2003, the first room-temperature stable inorganic electrides of [Ca24Al28O64]4+(e−)4 (termed C12A7:e−) was synthesized through generating two oxygen defects in 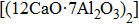
Based on the dimensionality of the anionic electrons and interstitial voids where the anionic electrons are confined, electrides can be classified into zero-dimensional (0-D), one-dimensional (1-D), two-dimensional (2-D), and three-dimensional (3-D) electrides. The representative 0-D electrides are C12A7,[5] Li12Mg3Si4,[28] and Ti2O,[29] in which the anionic electrons are trapped in atomic cages. The highly localized anionic electrons in 0-D electride lead to a narrow band in the band structure, termed as ‘interstitial band’ or ‘cage states’.[30] 1-D electrides are exemplified by Na3S,[31] [La8Sr2(SiO4)6]4+:4e−,[32] Y2Cl3,[7] Y5Si3,[33] and Cs3O,[34] in which the anionic electrons are distributed in the channel voids, forming 1-D electron gas. 2-D electrides are revealed in Ca2N,[27] YCl,[7] and Y2C,[35] in which the anionic electrons are trapped in 2-D interstitial voids having 2-D electron gas. In 2-D electrides, the anionic electrons having higher dimensionality exhibit much delocalized energy band in comparison with 0-D electrides. 3-D electrides are reported rarely; Li4N[36] and Ca2C[37] were reported theoretically to be 3-D electrides, however, 3-D electrides are still not confirmed in experiment.
High pressure is an effective way to discover or modify materials with intriguing physical and chemical properties.[38–42] Extensive theoretical and experimental efforts greatly enrich the variety of high-pressure electrides (HPEs). For example, elemental electrides of Li,[43–45] Na,[26,46] K,[45,47] Cs,[48] Al,[49] and C;[50] and new compounds of Na2He,[51] Ca5C2,[52] Li6P,[18] Na2K,[53] and Ti2O.[29] Under compression, moreover, some electrides undergo interesting physical and chemical alternations, such as metal-to-semiconductor transition in Ca2N.[54,55] Thus, exploring new electrides and their electronic properties under pressure is very interesting. In the past few years, advanced structural searching methodologies were used to explore new electrides,[56–59] which significantly accelerate the discovery of HPEs. The swarm-intelligence based CALYPSO structure searching method[60–62] is one of effective means to design and discover HPEs. In this review, we focus on the CALYPSO-assisted HPEs discovery to gain the basic understanding of discovery and characterization of HPEs.
Under strong compression, sodium is found to exhibit surprising ionic state when metal–insulator transition occurred over 100 GPa.[64] This intriguing phenomenon is confirmed by subsequent DFT calculations and experimental observations, and the proposed high-pressure phase of Na-hP4 is revealed to be an electride.[26,46,65,66] The insulating Na-hP4 nature is further verified by HSE06 calculations with a band gap of about 9.5 eV at 1.75 TPa.[66] Elemental electrides are also observed in Li,[43–45] K,[45,47] Cs,[48] Al,[49] and C,[50] under extreme compression up to 650 TPa. To understand the underlying origin of alkali metals under compression, Rousseau and Ashcroft[67] proposed the rc/rs parameters (positively correlated with pressure) to describe the compression ratio, where the rc is the core sphere of metals and rs is the Wigner–Seitz radius. When the rc/rs ratio increases, the electron charge density at the interstitial voids tends to deviate from the free electron gas, leading to the formation of localized electrons in the interstitial voids. In terms of band structure, the increased rc/rs ratio significantly reduces the band width, which leads to metal-to-semiconductor transformation under pressure.
To further interpret the formation mechanism of high-pressure electrides (HPEs), Miao and Hoffman proposed a unified theory of HPEs by treating electrons in the interstitial voids as interstitial quasi-atom (ISQ).[63,68,69] In the ISQ model, the energy levels of ISQ and valence orbitals of atoms vary significantly under pressure (Fig. 
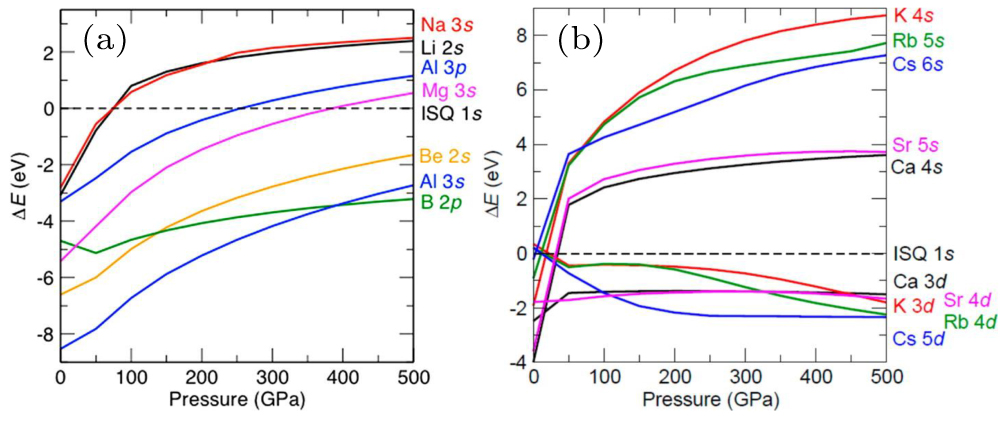 | Fig. 1. Pressure induced enthalpy variation of atomic valence orbitals compared with ISQ levels. Adapted with permission from Ref. [63] and full reference therein. |
Advanced structural searches, effective indicators for electrides, and high pressure experimental confirmations provide the great opportunities to quickly search and discover new HPEs. Here, the scheme for the exploration of new HPEs is illustrated in Fig.
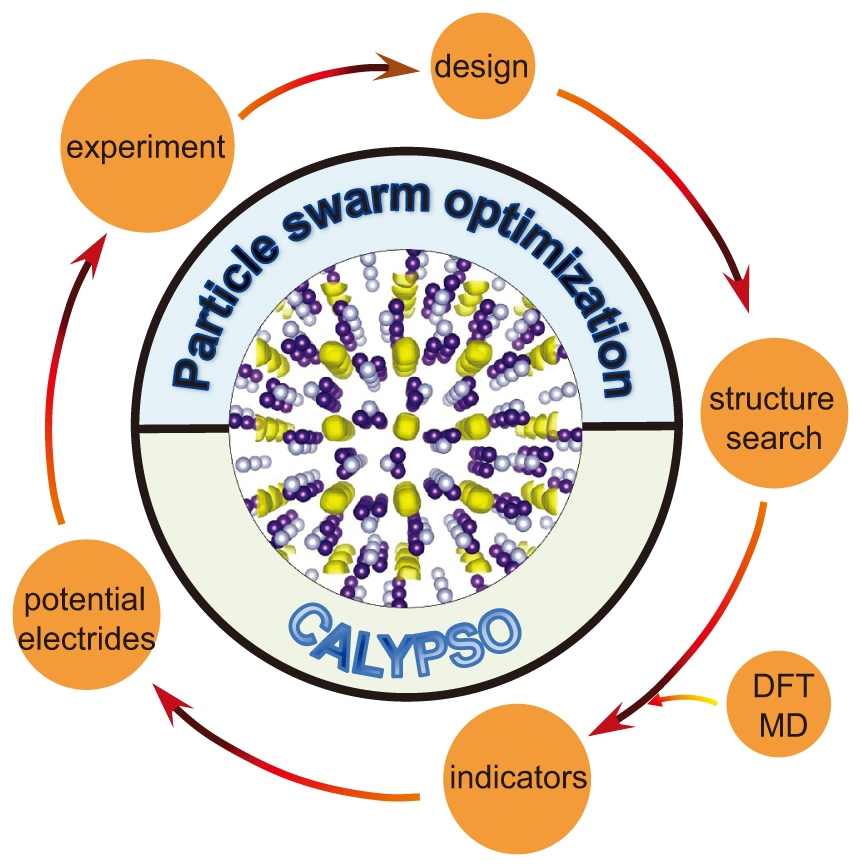 | Fig. 2. Scheme for exploring new HPEs from pre-design, CALYPSO assisted structural searching, indicators for electrides, to experimental synthesis. |
The searching of metal-rich stoichiometries with the possible charge imbalanced compounds is helpful to discover new electrides. Those compounds, which do not have intrinsically excess valence electrons, may also form electrides only when the anionic elements are aggregated (forming pairs or polyhedron) in the lattice to be the electron-rich systems. The constituted elements in the host structures are also found to have significantly effects on the formation of electrides. Using CALYPSO method, Zhang et al. carried out extensive structure search to explore electrides in the systems of A2B and AB (A = electrons donor, B = electrons acceptor).[37] 89 compounds were suggested to be potential electrides. It was also found that the electronegativity of elements is critical to the formation possibilities of electrides (proportion of electrides in all the generated phases within CALYPSO structural searches), since it is a direct measurement of electron affinity of an atom. For the anionic elements, the formation of electrides was surveyed in Ca2X systems (X = anionic elements) (Fig. 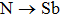
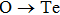
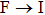
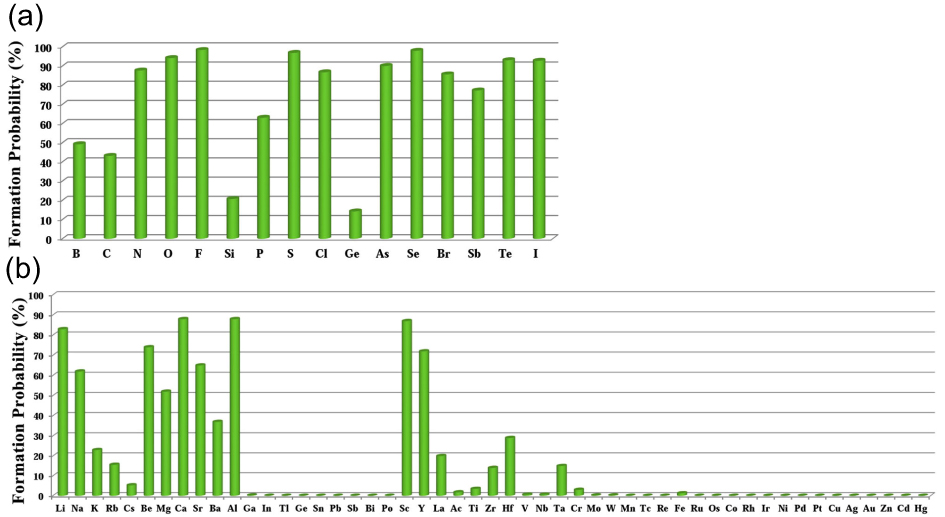 | Fig. 3. Possibility to form electrides in Ca2X systems (X = anionic elements) (a) and metal nitrides (b).[37]. |
Based on the constituted elements and compositions for pre-designed compounds, one can carry out extensive structural searches to explore the potential high-pressure electrides at specific pressure. Within CALYPSO method, the particle swarm optimization (PSO) algorithm has exhibited significant advantages in the electride searching, e.g., rapid convergence and few parameters to input.[61,70,71] Benefitting from these advantages, the CALYPSO method has made great success in exploring new HPEs, such as the noble gas containing electrides of Mg–NG (NG = Xe, Kr, Ar);[40] 3-D Li4N electride;[36] Ti oxides electride;[29] and superconducting Li6P electride (Tc=39.1 K).[18] To assist the experimental high-pressure phases for electrides, ‘X-ray diffraction data assisted structural prediction’ in CALYPSO code can significantly facilitate the materials discovery under pressure.[72]
Identifying an electride in practice is not an easy task, since the localized electrons in the interstitial voids are difficult to detect in experiment. Nevertheless, the ‘anionic electrons’ can be still inferred from the experimental methods, e.g., angle resolved photoemission spectroscopy.[73] On the other hand, theoretical calculation can provide a direct and feasible way to resolve the experimental challenges, which has been widely used to identify electrides. Although the excess valence electrons in host lattices can be found out through pre-design process; however, in some cases, the separation of the excess valence electrons and the metal–metal bonding is difficult in the generated compounds. Thus, the additional indicators to identify an electride are discussed below.
(i) Atomic voids. One of the most challenges to identify electrides is to separate the anionic electrons and metal–metal multi-center bonding. To form anionic electrons, the host structures need to have enough atomic voids to accommodate the excess electrons. Although the high electron density can also be observed in some interstitial voids, the relatively smaller interstitial voids preclude the formation of electride, due to the contributions of the metal–metal multi-center bonding. In our previous yttrium and scandium chlorides, the interstitial-voids topology is proposed for the discovery of potential electrides.[21] The F-center interstitial voids are most common interstitial voids, which can accommodate the excess electrons. In particular, it is valid for the transition metal containing inorganic electrides. In yttrium and scandium chlorides, there are five electrides identified, 2-D electrides of YCl and ScCl; 1-D electrides of Y2Cl3, Sc5Cl8, and Sc7Cl10. All these compounds possess the irregular metal octahedrons with large scale volumes, forming the layer-like (YCl and ScCl), channel-like (Y2Cl3 and Sc5Cl8) or double channel-like (Sc7Cl10) voids in the host structures. The nearest octahedrons also form a metal tetrahedron. High charge densities are observed in both the metal octahedrons and metal tetrahedrons. However, the smaller interstitial volume in the metal tetrahedrons cannot accommodate the anionic electrons, as we suggested. Detailed electronic analysis reveals that these electrons observed in the metal tetrahedrons stem from metal–metal π bonding; while the electrons in the metal octahedrons are confirmed to be the anionic electrons, which stays far away from the metal atoms and metal–metal bonds.
(ii) Electron localization function (ELF). ELF is an efficient way to measure the electron localization.[74,75] Highly localized electrons such as cores, bonds, and lone pairs have great ELF values close to 1, while the homogeneous electron gas is corresponding to the ELF value of 0.5. In electrides, the anionic electrons can be directly observed through the ELF analysis, since the highly localized interstitial electrons can result in the great ELF values that are far away from the nuclei and metal–metal bonds. Such criteria for electrides identifications is also suggested by Zhu et al.[76] They proposed that the ELF topology in electrides was characterized by the ELF attractor (non-center ELF maxima) in the interstitial voids. Through the ELF analysis, the inorganic electride of C12A7:2e− was identified theoretically. Martinez-Canales et al. also identified the high pressure electrides carbon in FCC lattice by checking the ELF maxima in the vacancy voids.[50] Subsequently, great ELF values off the nuclei have been widely used to describe electrides.
(iii) Density of states (DOS) and band structure. Anionic electrons have high charge density in the interstitial voids, which can be reflected in the DOS and band structure. In the band structure, the anionic electrons may be distributed in the interstitial bands or projected onto the bands of the neighboring real atoms, e.g., LaCoSi, LaRuSi, and CaRuSi.[20,77] Through the visualization of the electron density in these bands, where the anionic electrons were confined, one can unambiguously distinguish the distributions of the anionic electrons. Analogy to the ELF attractors, high charge density staying away from the ions and bonds can also be observed in the interstitial voids. Moreover, in the energy range (in the DOS curves) where the anionic electrons are distributed, the contribution of anionic electrons to DOS can be separated quantitatively, which is much higher than other atomic orbitals.
The theoretical predictions of HPEs can provide a guide to experimental synthesis. Many HPEs were predicted and synthesized successfully, such as Na,[26] Ca2N,[54,55] Sr5P3,[57] and Na2He.[51] The metal-to-semiconductor transition in Ca2N is also predicted under pressure. Two semiconducting high-pressure phases of I-42d- and Cc-type Ca2N was predicted to be 0-D electrides, and these two electrides are found in later experiment. In our recent work, we directly observe such metal-to-semiconductor transition under pressure (Fig.
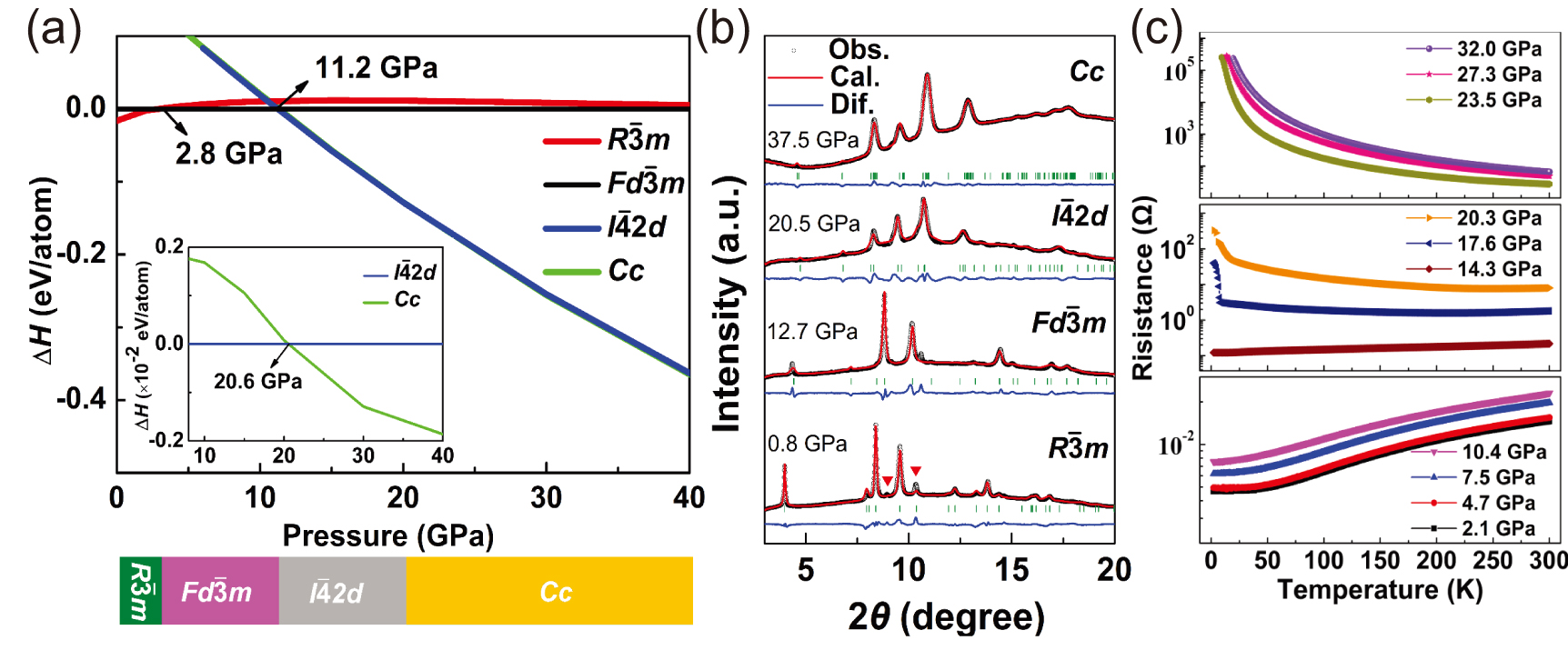 | Fig. 4. (a) Predicted structural transformations of Ca2N under pressure. (b) Rietveld refinements of XRD patterns of Ca2N in R-3m, Fd-3m, I-42d, and Cc lattices. (c) Measured electrical resistance as a function of temperature at different pressure.[55]. |
High pressure has significant effects on the distribution of anionic electrons. As proposed by Rousseau and Ashcroft, the increased pressure can weaken the connections between interstitial electrons and free electron gas.[67] On the other hand, the high-pressure phases usually take closely packed arrangement compared with the ground state structures. Consequently, the volume of interstitial voids can decrease significantly. When compressing an electride, the electronic dimensionality of electrides is usually reduced. Such phenomenon has been verified in the high-pressure investigation of Ca2N,[55] a 2-D electride under ambient condition. As shown in Fig.
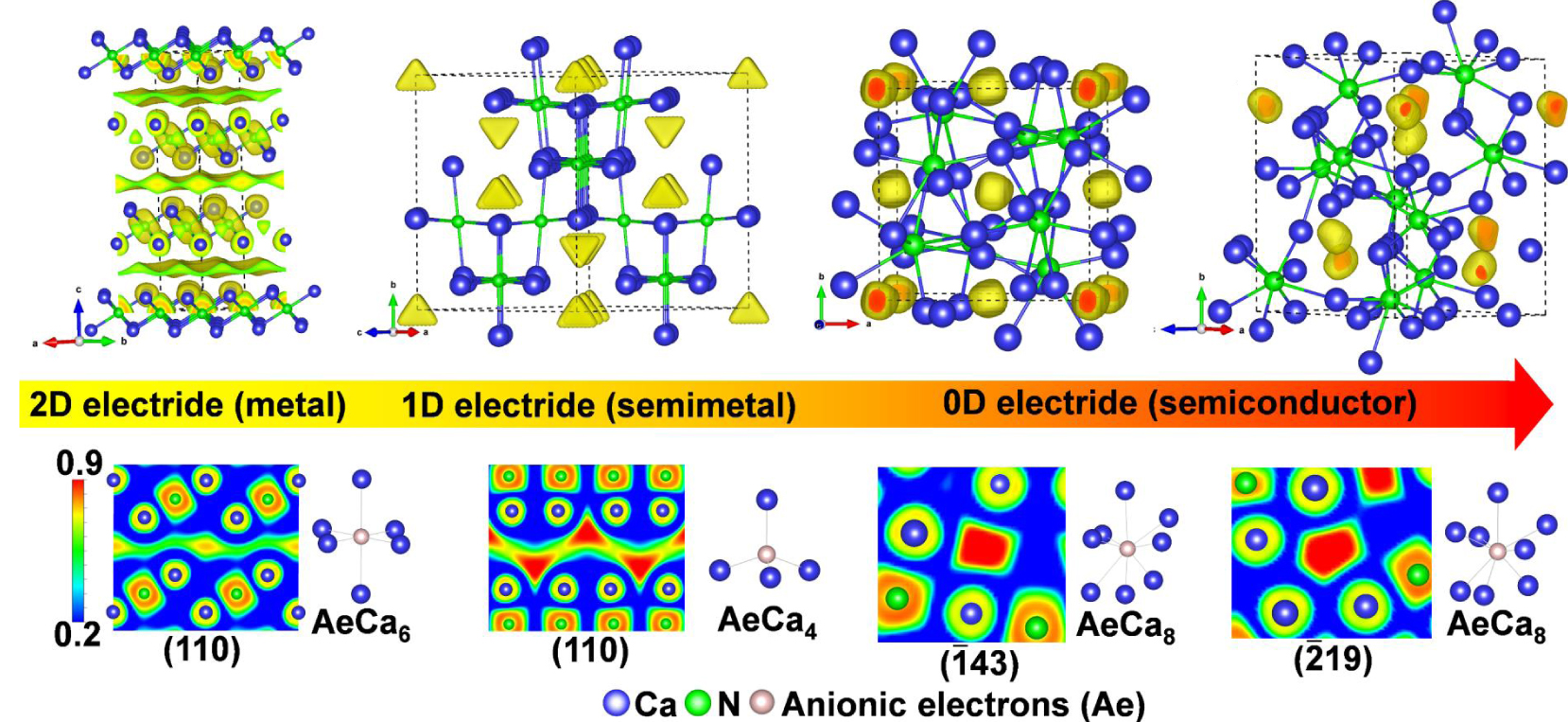 | Fig. 5. Reduction of electronic dimensionality in Ca2N under pressure: from 2D-, 1D-, to 0D electrides.[55]. |
Despite of many successful predictions for HPEs, there are few of them synthesized in experiments, because of the following several reasons: (i) Many predicted HPEs can only be stabilized under ultra-high pressure, such as metal N electride at TPa pressures,[78] Li6P at 178 GPa,[18] and Mg2O3 at 500 GPa,[41] which is difficult to be synthesized based on current experimental approach. (ii) Most of HPEs are unquenchable to ambient conditions, which limits further characterizations and applications. (iii) Many inorganic electrides are air and water sensitive. For example, electrides possess strong hydrogen affinity,[57,79,80] caused by the small atomic radius and high electronegativity of H atoms. (iv) Under compression, many systems such as Li–S,[81] Li–P,[82] and Li–Na[83] show quite rich stoichiometries; moreover, the relative energy differences between different compositions are very small, e.g., Li4N.[36] In practice, it is very difficult to separate and characterize these potential HPEs due to the phase coexistence and competition.
In summary, we overview the formation mechanism, design principles, structural searching assisted by CALYPSO method, theoretical indicators to identify electrides, and experimental synthesis of HPEs. We discuss the structural changes and electronic dimensionality evolution under compression of electrides under compression. Despite of the successes of electride investigations, the obtained HPEs in experiment are still rare, the realization of quenchable HPEs and exploration of the utilizations of HPEs remains open.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] | |
| [62] | |
| [63] | |
| [64] | |
| [65] | |
| [66] | |
| [67] | |
| [68] | |
| [69] | |
| [70] | |
| [71] | |
| [72] | |
| [73] | |
| [74] | |
| [75] | |
| [76] | |
| [77] | |
| [78] | |
| [79] | |
| [80] | |
| [81] | |
| [82] | |
| [83] |

