† Corresponding author. E-mail:
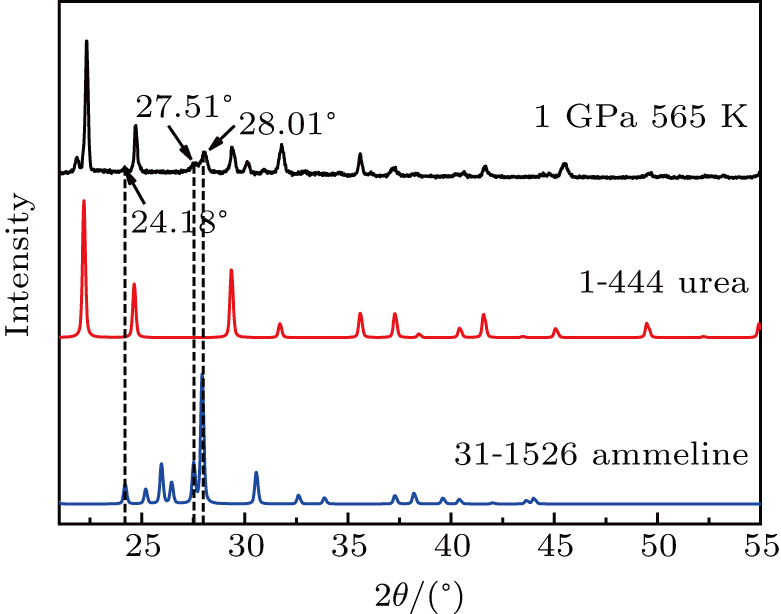
The properties of urea under high pressure and high temperature (HPHT) are studied using a China-type large volume cubic high-presentation apparatus (CHPA) (SPD-6 × 600). The samples are characterized by scanning electron microscopy (SEM), x-ray diffraction (XRD), and Raman spectroscopy. By directly observing the macroscopic morphology of urea with SEM, it is confirmed that the melting point of urea rises with the increase of pressure. The XRD patterns of urea residues derived under different pressures show that the thermal stability of urea also increases with the increase of pressure. The XRD pattern of the urea residue confirms the presence of C3H5N5O (ammeline) in the residue. A new peak emerges at 21.80°, which is different from any peak of all urea pyrolysis products under normal pressure. A more pronounced peak appears at 708 cm−1 in the Raman spectrum, which is produced by C–H off-plane bending. It is determined that the urea will produce a new substance with a C–H bond under HPHT, and the assessment of this substance requires further experiments.
Urea is a simple organic compound and is the main nitrogen-containing end product of protein metabolism in mammals and certain fish.[1] The German chemist Friedrich Wöhler disproved the vitalistic theory by synthesizing urea in a lab. Today, urea is widely used as an industrial raw material, and its derivatives are used in fertilizers.[2–7] Urea is also a good organic catalyst,[1,3,4] and in recent years it has been used in the synthesis of mesoporous spinel NiCo2O4 nanostructures,[5] hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity,[4] and nitrogen-doped graphene hydrogels.[8] The pyrolysis reaction of urea under normal pressure has been comprehensively studied, however, the characteristics of urea under high pressure have not yet been investigated. When the temperature reaches the decomposition point of urea, the urea will decompose to produce ammonia and cyanic acid, and the decomposition rate will increase with the increase of temperature. As the temperature in the reactor continues to rise, the cyanic acid reacts with the urea to form a biuret. The biuret will then react with the cyanic acid to produce cyanuric acid. The urea in the reactor will reacts with the pyrolysis product, and the pyrolysis products will also react with each other, which causes the pyrolysis reaction of urea to become particularly complicated.[9,10] Studying the properties of urea under high pressure and high temperature (HPHT) is also beneficial to expand the use of urea under high pressure conditions.
Urea is a ubiquitous organic matter composed of C, H, O, and N elements in the earth’s crust. The study of the characteristics of urea under high temperature and high pressure will also provide data support for researchers exploring forms of urea existing in the earth and its possible reactions. According to the earth science research, the pressure at 35 km below the surface is about 1.0 GPa.[11–13] From this, it can be estimated that the deep pressure of the lithosphere is about 1.0 GPa. Thus, the pressure interval is determined to be between 0.3 GPa and 1.0 GPa according to the high temperature and high pressure measurement equipment.
In this paper, urea is studied under high temperature and high pressure. It is observed that the melting point and thermal stability of urea will increase with the increase of pressure. To a certain extent, this discovery explains the characteristic change of urea under high pressure, and provides data support for researchers to study the state of different organic compounds under HPHT. At the same time, urea exhibits different atmospheric pressure characteristics under HPHT, which is conducive for expanding its industrial use.
High temperature and high pressure testing of urea is carried out using a China-type large volume cubic high-pressure apparatus (CHPA) (SPD-6 × 600) in a pressure range of 0.3–1.0 GPa and a temperature range of 300–600 K. The experimental assembly is provided in Fig.
The purity of the urea used is 99.0 wt.%. Before testing, the urea is placed in an agate mortar for thorough grinding to ensure uniform particle size. This is advantageous for observing the change in morphology of the urea under SEM and determining if the urea melts.
The temperature measurement assembly of the experiment is illustrated in Fig.
Once the experiment is complete, whether the urea has leaked is directly observed to determine the melting point of the urea. The morphology of the sample surface is observed using a scanning electron microscope (SEM), the residue of urea is analyzed by x-ray diffraction (XRD), and Raman spectroscopy is used to determine the product of urea at high temperature and high pressure.
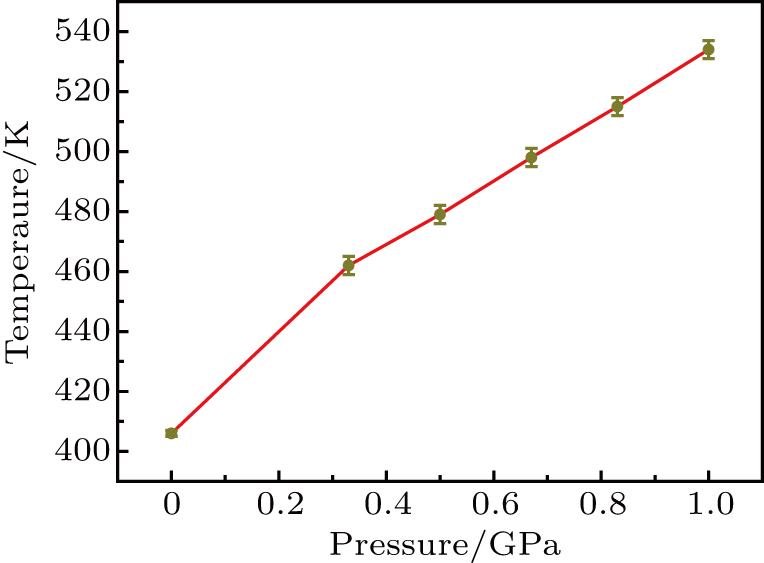
Urea is a molecular crystal with a melting point of 405.7 K under normal pressure.[16] Table
| Table 1.
Melting point experiment of urea under different temperature and pressure conditions. . |
The assembly diagram of the experiment is shown in Fig.
Figure
Once the the pyrophyllite is removed, the residue of urea in the reaction chamber can be observed. The urea residue in Fig.
Both R-3 and R-2 have the same pressure and only the experimental temperature is reduced, however, the urea cannot permeate from the cavity. Even when the experimental time is extended to 90 min, the urea does not flow out of the chamber. This proves that urea will only flow out of the chamber when the temperature reaches the melting point. If the melting point is not reached, even if the time is extended, the urea will not melt. According to these results, it can be determined that the melting point of urea is 480 K at the pressure of 0.5 GPa.
A temperature–pressure map is provided in Fig.
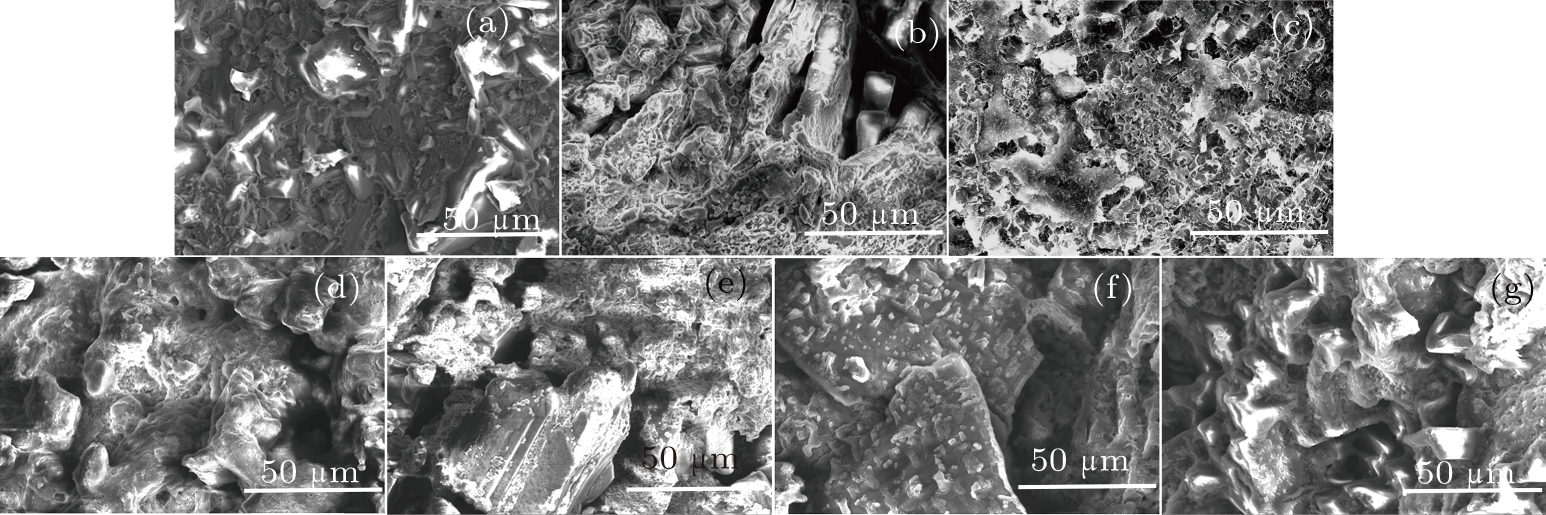
The melting point of urea can be determined by the phenomenon in which the urea permeates out of the cavity after melting. As the experimental chamber is sealed, the process of melting the urea in the chamber cannot be observed in real time. The experiment time is thus shortened in order to capture the urea in the middle of the melting process. The melting process of urea is then determined by observing its microscopic morphology.
A scanning electron micrograph of R-7 cold pressed urea is provided in Fig.
A scanning electron micrograph of the R-1 residual sample is provided in Fig.
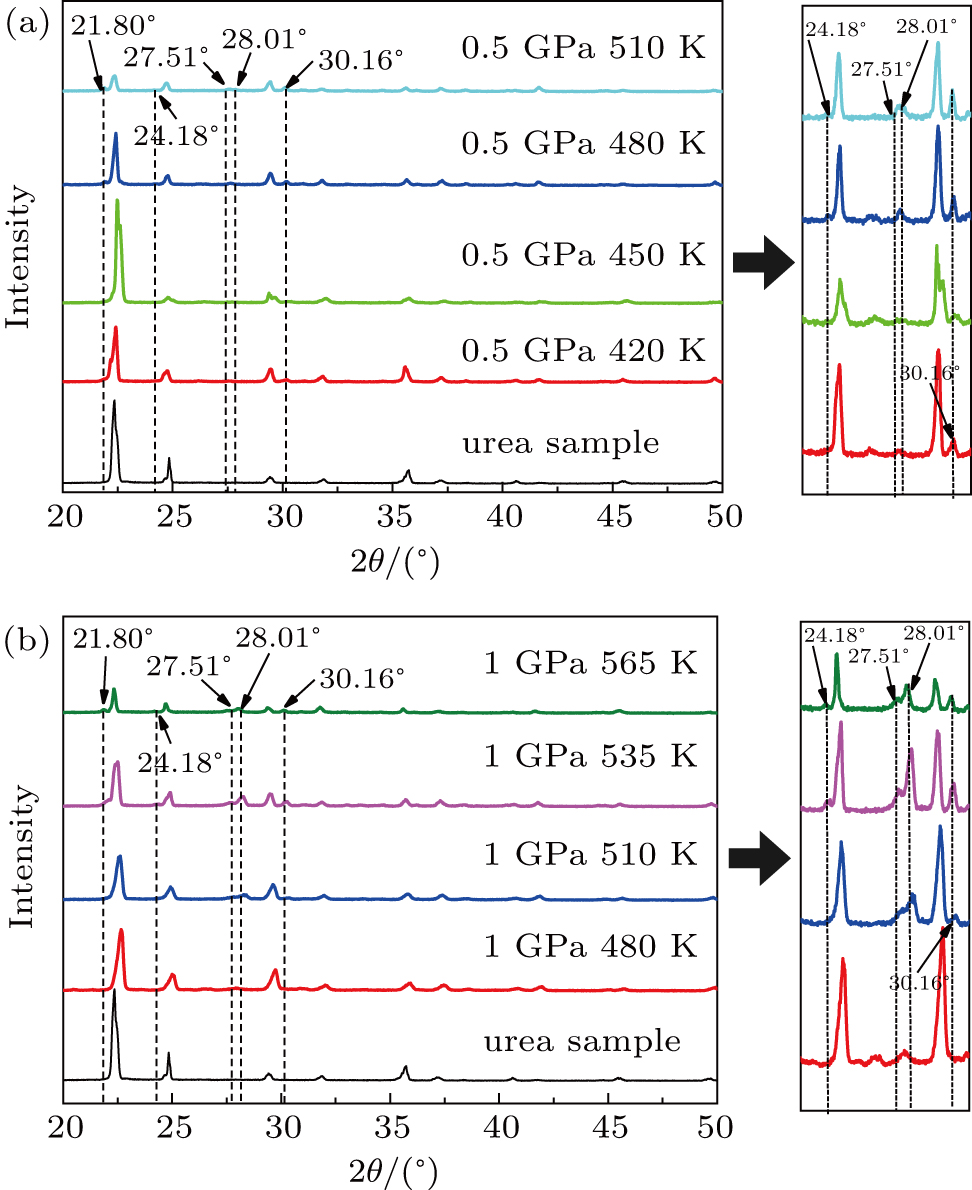
The XRD pattern of residual urea at different temperatures is shown in Fig.
As shown in Fig.
The XRD pattern of residual urea at different temperatures at a pressure of 1 GPa is illustrated in Fig.
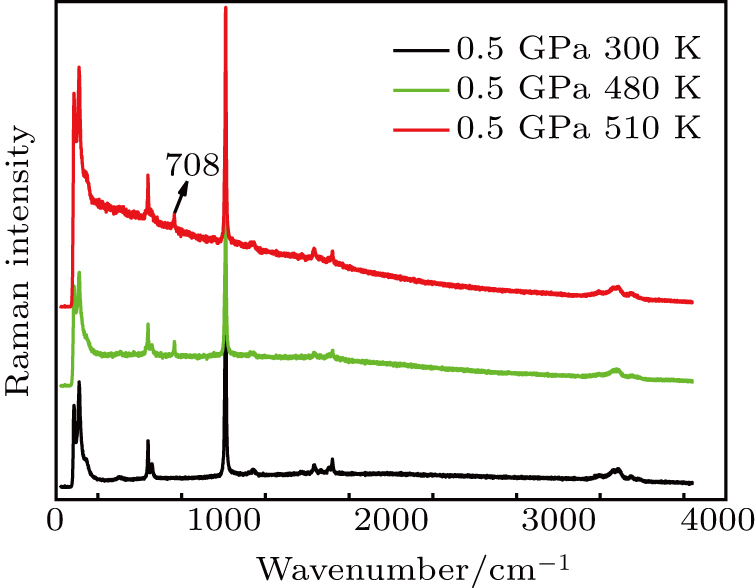
The Raman spectra of residual urea at different temperatures under the same pressure conditions are provided in Fig.
In this study, it was determined that the melting point of urea rises with the increase of pressure and is proportional to the pressure. The XRD analysis of urea residues at different temperatures and pressures illustrated that the thermal stability of urea increases with the increase of pressure. The product of urea under high temperature and high pressure is C3H5N5O, which can also be produced in the pyrolysis process of urea under atmospheric pressure. With further increase of temperature, XRD spectra illustrated that new substances are produced at different atmospheric pressures. When the pressure is involved in urea reactions, new substances are also produced. The different characteristics of urea under high temperature and high pressure are conducive to the expansion of its application and further development in various industrial and energy fields.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] |