† Corresponding author. E-mail:
The multi-walled carbon nanotubes (MWCNTs) studied in this work were synthesized by the catalytic chemical vapor deposition (CCVD) process, and were thermally annealed by the hot filament plasma enhanced (HF PE) method at 550 °C for two hours. The x-ray absorption near edge structure (XANES) technique was used to investigate the adsorption and desorption phenomena of the MWCNTs at normal and grazing incidence angles. The adsorbates were found to have different sensitivities to the thermal annealing. The geometry of the incident beam consistently gave information about the adsorption and desorption phenomena. In addition, the adsorption of non-intrinsic potassium quantitatively affected the intrinsic adsorbates and contributed to increase the conductivity of the MWCNTs. The desorption of potassium was almost 70% greater after the thermal annealing. The potassium non-intrinsic adsorbates are from a physisorption mechanism whereas the intrinsic adsorbates result from chemisorption.
The theoretical and experimental knowledge of the structure of a system is required to elucidate new materials with novel properties. Carbon nanotubes (CNTs) have intensively undergone theoretical and experimental studies because of their versatilities. During the first decade of the discovery of CNTs by Sumio Iijima, they were usually obtained at high temperature (about 3500 °C) using graphite electrodes by the electric discharge process.[1] Nowadays, they are produced in industries at intermediate temperatures (between 700–950 °C) by the well-known catalytic chemical vapor deposition (CCVD) process,[2,3] electric discharge,[1] or laser ablation.[4] Among these, CCVD is one of the most suitable methods for synthesizing aligned CNTs since it can be carried out at low temperatures and pressures.[5] However, CCVD synthesized CNTs usually contain catalytic metallics and impurities whose nature and proportion depend on the synthesis parameters. The presence of impurities can significantly affect the properties of CNTs and consequently the global behavior of these materials.[6] The samples studied herein are multi-walled CNTs (MWCNTs) synthesized by the hot filament plasma enhanced catalytic chemical vapor deposition (HF PE CCVD) process fully described in Ref. [7]. CNTs are mostly characterized by scanning electron microscopy (SEM)[8] and transmission electron microscopy (TEM),[9] which are both qualitative methods. These techniques do not give enough information about the electronic and structural properties of the CNTs. The x-ray absorption near edge structure (XANES) technique on the contrary is a powerful tool that provides the structural and electronic information on the local environment around the absorber atom in the medium range order due to its angular dependence of the absorption transition.[7] This technique is sensitive to the chemical adsorption and impurities, defects, and orbital rehybridization.[7] MWCNTs with large diameter are advantageous for various applications like molecular compounds for instance, in organic solar cells,[10] infrared detectors,[11] and biological systems.[12] The adsorption and desorption phenomena of the MWCNTs are of great interest since they provide a full understanding of the performance for water and wastewater treatment applications.
In this study, through XANES technique, we investigate the adsorption and desorption phenomena of MWCNTs grown by CCVD process and thermally annealed at 550 °C. The XANES spectra are collected at normal incidence (NI) and grazing incidence (GI) angles to evaluate the adsorption percentage.
MWCNT is made up of a bundle of single walled CNTs (SWCNT) arranged such that they all have the same vertical axis. The external diameter of the MWCNT depends on the growth process. By the electric arc discharge, the external diameter is about 20 nm, whereas it can reach 100 nm with the CCVD process.[2] In general, as a consequence of the synthesis process, the surfaces of the CNTs are usually covered by some adsorbates mostly considered as impurities.[13] These adsorbates can affect, in different ways, the structure of the surfaces since they can be linked physically by van der Waals forces or chemical covalent bonds.[13] The former is named physisorption mechanism while the latter is chemisorption. If the free bonds are saturated, the strongly linked adsorbates can either eliminate or favor the restructuring of the free surface, which thus may or may not affect the properties of the surface.[13] It has been established that the Lennard–Jones interactions prevail in the adsorption mechanism,[14] suggesting that the interaction potential V between two species with a distance r can be written as a sum of the terms of the repulsion and attraction contributions, as follows:
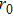
Chemisorption is a dominant process in SWCNTs.[15] On the contrary, physisorption, which is a characteristic of MWCNTs, is a reversible process that can occur at low temperatures.[15] Adsorption capacity depends on the specific surface area and the number of walls in the MWCNT. The specific surface area reduces with an increase in diameter and in the number of walls.[16] The average specific surface area of the MWCNTs is between 30 m2/g and 400 m2/g depending on the mean diameter and the number of walls.[16] The specific surface area S of a MWCNT is given by[16]
The XANES spectra collected at the carbon K edge are shown in Figs. 






 | Fig. 1. Experimental and simulated XANES spectra of MWCNTs annealed and without contaminants at (a) normal and (b) grazing incidences. |
 | Fig. 2. Experimental and simulated XANES spectra of MWCNTs annealed and contaminated with potassium at (a) normal and (b) grazing incidences. |
Tables
| Table 1.
Main feature parameters for annealed and potassium-contaminated MWCNTs from XANES spectra at normal and grazing incidence angles. The intensity is in units of counts per second. . |
| Table 2.
Main feature parameters for unannealed and potassium-contaminated MWCNTs from XANES spectra at grazing and normal incidence angles. The intensity is in units of counts per second. . |
Each adsorbate corresponds to a specific electronic transition which is assigned to peaks 

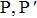
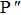
The individual contributions of the adsorbates present in our samples exhibit significant differences at normal and grazing incidence angles. During the adsorption process, rehybridization can occur in which the sp2 and sp3 orbitals are mixed leading to two free hybrid orbitals. Such that, if the contribution of sp3 increases, this will lead to the presence of more free bonds, resulting in the increase of the reactivity.
Figure
 | Fig. 3. The trend of the intensities of the adsorbates for the contaminated un-annealed and annealed MWCNTs at normal and grazing incidence angles. |
Comparing the intensities of the annealed and un-annealed contaminated MWCNTs shows that the non-intrinsic adsorbates 



It can be observed that potassium adsorbates (



The characterization of the carbon nanostructures after synthesis is mandatory since the properties of these materials can be well understood for applications related to adsorption and desorption. These nanostructures can then be applied to solve problems of water, air, and soil purification. XANES spectroscopy was used to qualitatively and quantitatively evaluate the adsorbed species on the MWCNTs. We observed that the quantity adsorbed on the surface is proportional to the diameter of the multi-walls. In addition, the incident angle of the incoming beam plays a key role in understanding the adsorption phenomenon. The results showed that the potassium non-intrinsic adsorbates are resulted from the physisorption mechanism whereas the intrinsic adsorbates are from chemisorption. Also, the intrinsic adsorbates are less sensitive to the thermal treatment compared to the non-intrinsic potassium adsorbates. In addition, it is seen that the presence of potassium increases the conductivity of the MWCNTs after thermal treatment, and the desorption process is mostly affected at grazing incidence. It is worth noting that the adsorbates are useful for non-covalent functionalization with potential applications in electronic, biologic, water treatment, and environmental protection. Moreover, thermal annealing is one of the efficient methods for the purification and controlling the conductivity of the MWCNTs.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] |