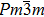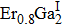† Corresponding author. E-mail:
ErGa3−xMnx disordered alloy is successfully prepared by the vacuum arc melting technology, and the crystal structure and magnetic properties are investigated by using the x-ray diffraction and magnetic measurements. The Rietveld structural analysis indicates that the ErGa3−xMnx crystallizes into a cubic structure with space group of 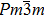


Most of RX3 intermetallic compounds (R = rare earth; X = In, Ga, Tl, Sn, and Pb) have a cubic AuCu3-type crystal structure.[1–8] The interest in RX3 is growing due to their salient features such as formation of magnetic moment, crystal field effect, and multiaxial magnetic structures.[9–11] In the last decade, the research activities have focused on the binary intermetallic compounds RIn3 with cubic AuCu3-type structure due to their excellent physical properties such as low temperature superconductor, complex magnetic ordering, Kondo effect, and multi-axis magnetic structure.[12–14] Gallium belongs to the IIIA group, but the RGa3 intermetallics cannot be formed between the light rare earth and Ga. In the heavy rare earth case, the stoichiometry RGa 1:3-type intermetallics only exist in a few of rare earths such as: Tm, Lu, Dy, Ho, and Er,[15,16] where TmGa3, LuGa3, and ErGa3 crystallize into a cubic AuCu3-type structure.[17] TmGa3 shows a modulated antiferromagnetic structure at low temperature, it orders antiferromagnetically around 4.2 K.[18] The saturation moment of TmGa3 is 

As is well known, the 3d transition metal Mn plays an important role in magnetic materials due to its significant contribution to the magnetic anisotropy by coupling with 4f electrons of the rare-earth.[19–21] Doping Mn into GdIn3 leads to a strong correlation between electric transport and magnetic state, and the electric transitions are associated with the magnetic transitions due to the Stoner spin fluctuation with the increase of temperature. In this study, Mn-diluted ErGa3 magnetic alloy is designed for providing the possibility of the exchange interaction between 3d electrons of Mn and 4f electrons of Er and for expecting to modulate the magnetic characteristics of Mn diluted in ErGa3 magnetic alloy.
The samples were prepared according to the normal structural formula of ErGa3−xMnx (x = 0–0.3) and the purities of raw metals were higher than 99.9 wt%. Owing to the evaporation loss of Er and Mn in the melting process, their weights are appropriately compensated for by supplying extra contents for Er: 1%∼2% and Mn: 8%∼10%. The samples were melted in the non-consumable vacuum arc furnace under atmosphere of high pure argon gas. Each sample was re-melted four times to ensure the homogeneity, and followed by being annealed at 800 °C for 168 h in the evacuated quartz tube in order to eliminate the residual stress, and finally by quenching in water.
The crystal structures of samples were determined by x-ray diffraction (XRD) with Cu–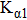
The field dependence of magnetization curves (M–H) were measured with a vibrating sample magnetometer (VSM) under the applied fields ranging from −20 kOe (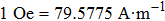
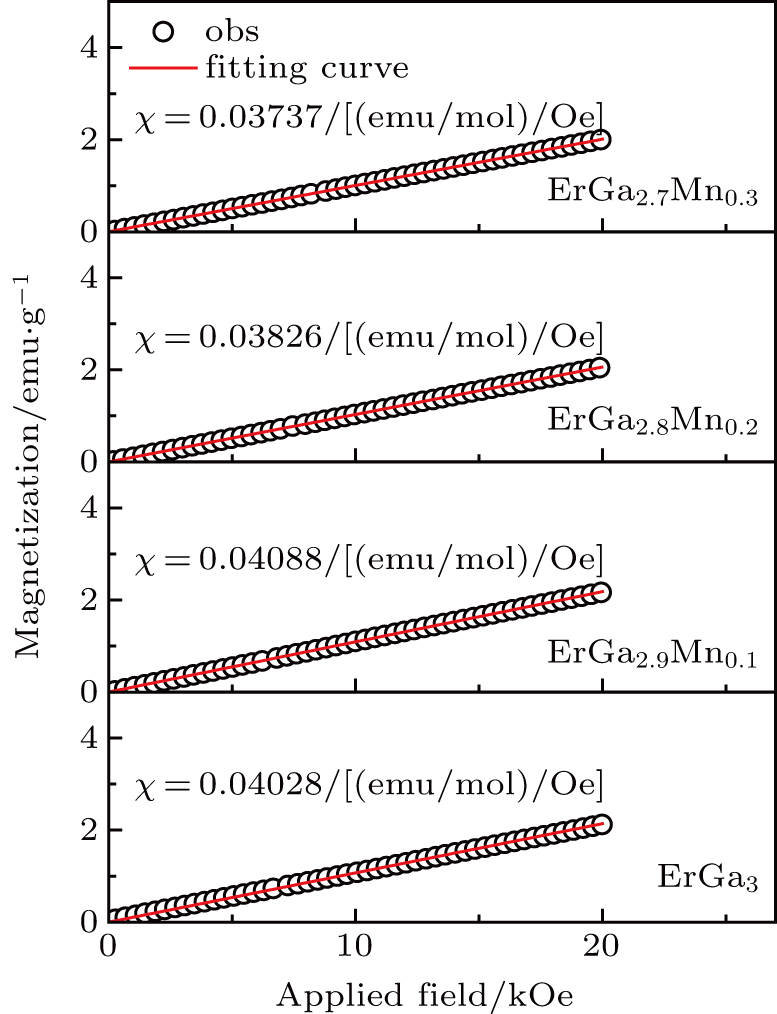
As shown in Fig.
 | Fig. 1. XRD patterns of (a) ErGa3−xMnx alloys and (b) enlarged patterns in 2θ ranging from 32° to 38°. |
| Table 1.
Lattice parameters of cubic ErGa3−xMnx alloys. . |
The crystal structure of ErGa3−xMnx was refined by using the Rietveld refinement method. Figures 


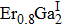

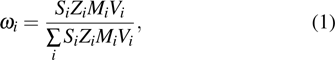 |
 | Fig. 2. Observed and calculated XRD patterns of ErGa3−xMnx for (a) x = 0, (b) x = 0.1, (c) x = 0.2, and (d) x = 0.3. |
| Table 2.
Rietveld refinement structural parameters of ErGa3−xMnx. . |
| Table 3.
Rietveld refinement structural parameters of |
The bond lengths in ErGa3−xMnx intermetallic compounds are calculated according to the refined atomic coordinates and the results are shown in Figs.
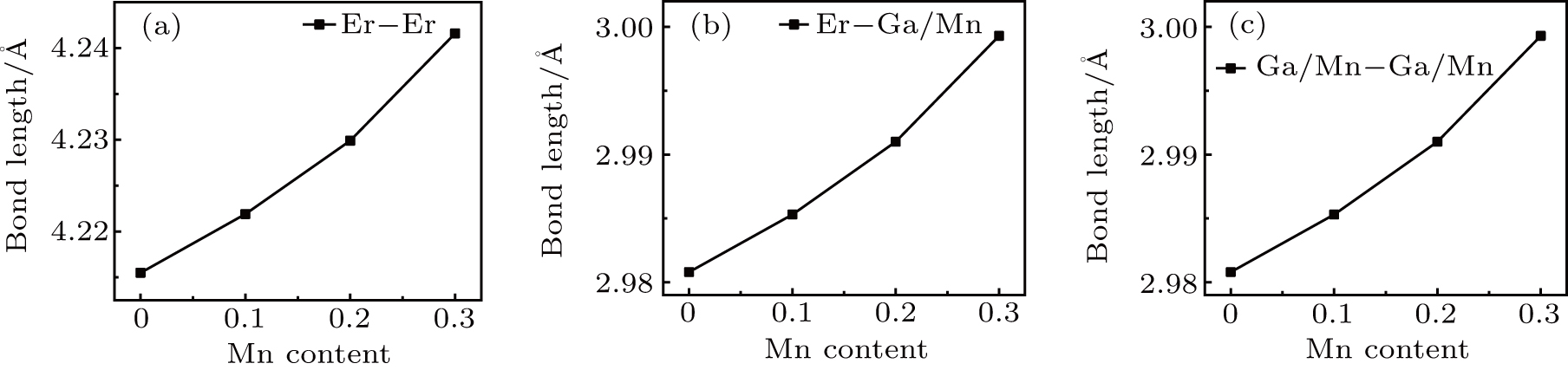 | Fig. 3. Mn content-dependent bond lengths of ErGa3−xMnx compounds for (a) Er–Er, (b) Er–(Ga, Mn), and (c) (Ga, Mn)–(Ga, Mn). |
 | Fig. 4. Projections of cubic ErGa3 along [111] direction and hexagonal ErGa2(Ga, Mn)0.4 along [001] direction for (a) cubic structure and (b) hexagonal structure. |
| Table 4.
Bond lengths of cubic ErGa3−xMnx. . |
Figure
 |
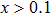
 | Fig. 6. Curves of Mn content-dependent fitted parameters of ErGa3−xMnx for (a) lattice parameter a and volume V, and (b) susceptibility χ. |
| Table 5.
Fitted parameters of ErGa3−xMnx. . |
The Mn doped ErGa3−xMnx alloys crystallize into cubic structure with a space group of 

The ErGa3−xMnx alloys show paramagnetic characteristics at room temperature. With the increase of Mn content in a range of x = 0–0.1, the magnetic susceptibility χ increases due to the Mn ion and Er ion increasingly contributing to effective moment. However, the magnetic susceptibility χ decreases with Mn content increasing, which is due to the phase separation from the cubic Er(Ga, Mn)3 to Er0.8Ga2(Ga, Mn)0.4. The lattice distortion is the phase transition driving force and causes the Er–Er and Mn–Mn bond lengths to be prolonged, which induces the susceptibility to decrease.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 |