† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 11875210), China Postdoctoral Science Foundation (Grant No. 2018M640724), the International Cooperation Program of Guangdong Provincial Science and Technology Plan Project (Grant No. 2018A050506082), and the Talent Project of Lingnan Normal University, China (Grant No. ZL1931).
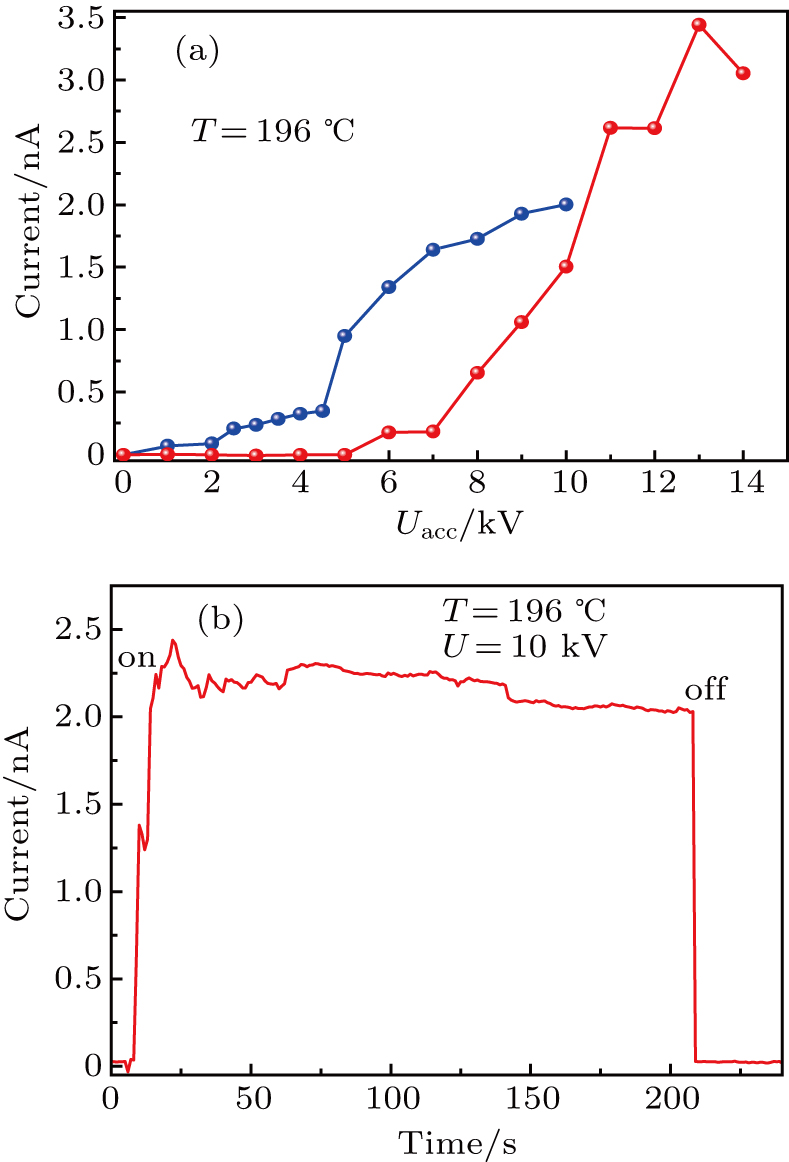
We fabricated a silver ion emitter based on the solid state electrolyte film of RbAg4I5 prepared by pulsed laser deposition. The RbAg4I5 target for PLD process was mechano-chemically synthesized by high-energy ball milling in Ar atmosphere using β-AgI and RbI as raw materials. The ion-conducting properties of RbAg4I5 were studied by alternating current (AC) impedance spectroscopy and the ionic conductivity at room temperature was estimated 0.21 S/m. The structure, morphology, and elemental composition of the RbAg4I5 film were investigated. The Ag+ ion-conducting property of the prepared superioni-conductor film was exploited for ion–beam generation. The temperature and accelerating voltage dependences of the ion current were studied. Few nA current was obtained at the temperature of 196 °C and the accelerating voltage of 10 kV.
Solid state electrolytes (SSEs) represent a class of materials with ionic conductivity owing to the fast transport paths for the mobile ions inside them. In 1961, Reuter et al. reported SSEs of Ag3SI and Ag3SBr with the ionic conductivity of 10−2 S/cm at 25 °C, which breaks the restrictions on the application of SSEs because of their high ionic conductivity (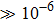
In addition, SSEs are promising candidates in field emission electric propulsion (FEEP) systems, due to their superior features of compactness, long working life, and low power consumption.[16,17] FEEP systems with SSEs can be used on space-constrained micro-spacecraft to enable on-orbit controls such as raising, lowering of its altitude and repositioning of the spacecraft owing to the ability of the source to produce controllable thrust within 
RbAg4I5 was prepared and investigated in the mid-1960s.[18] In 1967, Owens et al. revealed that crystalline compound RbAg4I5 exhibited superior ion conductivity of more than 101 S/m at 25 °C with negligible electronic contribution of 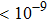
In the present work, the RbAg4I5 solid state electrolyte film was deposited at the apex of a silver needle by pulsed laser deposition (PLD), constituting an Ag+ ion emitter. The ion-conducting properties of RbAg4I5 solid electrolyte were studied. The characteristics of the point-like ion source were tested and analyzed. As an advanced type of ion emissive device, solid electrolyte ion sources are promising candidates for use in the ion propulsion system of miniature spacecraft with limited on-board payloads.
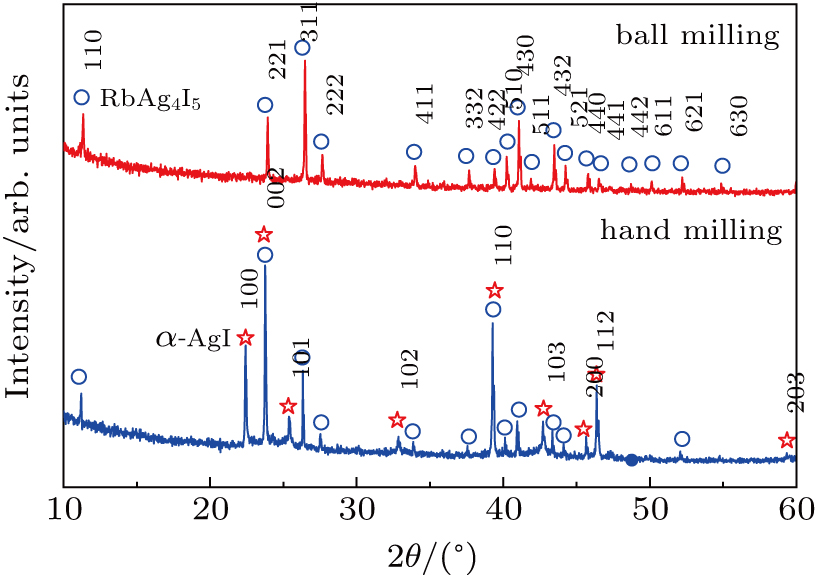
RbAg4I5 was synthesized by high-energy ball milling process in Ar atmosphere. Pure powders of β-AgI and RbI were used as raw materials. Desired amounts of β-AgI and RbI (molecular ratio was 4:1) were weighed and mixed in an agate mortar. First, the mixture was ground by an agate pestle for 5 min beforehand to obtain the homogeneous reactants. Then, the mixture powder with numerous 6-mm diameter agate balls was placed in an agate container of 100 ml in volume for milling. Next, the agate container was placed in a stainless steel vacuum sleeve with a hole for pumping and feeding argon. The mechanical milling process was performed in a planetary ball mill (DY-30, Nanjing NANDA), which was rotated at a speed of 380 rpm for 2.5 h. Finally, the ball-milled powders were annealed in argon at 150 °C for 5 h.
The RbAg4I5 thin film was deposited on the apex of a Ag tip and simultaneously on the Ag substrate by a pulsed laser deposition (PLD) system (PLD-450b, Germany COHERENT). The synthesized RbAg4I5 powders were pressed into a pellet (target) with a diameter of 30 mm and thickness of 1 mm–2 mm. A KrF laser (GCR-170, Spectra Physics) operated at the wavelength of 248 nm with the maximum energy-per-pulse of 200 mJ was used for irradiation in the pulsed laser system. The laser pulse width and pulse frequency are 30 ns and 5 Hz, respectively, and the laser average power is 0.7 W. The laser beam reflected from a reflective mirror was align to focus on a small zone of the target surface (a circular light spot with diameter of 1 mm–2 mm). The target-substrate distance was 55 mm. The pressure within the vacuum chamber was kept to be 4×10−3 Pa. The target was rotated at a speed of 10 rpm driven by a motor. The deposition was carried out for 50 min at the substrate temperature of 80 °C.
The RbAg4I5 powder was pressed into a pellet with diameter of 6 mm and thickness of 1 mm–2 mm by an electric press machine (DY-30, Tianjin KEQIGAOXIN), which supplied a molding pressure of 6 MPa for 1 min, then silver electrodes were deposited on both sides of the pellet. The pellet was used for AC impedance measurements, which was conducted from 20 Hz to 1 MHz at various temperatures using an impedance meter (6500B, Wayne Kerr).
The RbAg4I5 powder and thin film were characterized by x-ray diffraction (XRD) (D8 Advance, BRUKER) using Cu-Kα radiation (λ = 0.1542 nm) within the 2θ range of 20°–60°. The morphology of the RbAg4I5 thin film was observed by SEM (SIRION, FEI) equipped with EDS. The elemental composition of the RbAg4I5 solid electrolyte film was investigated by XPS (ESCALAB 250 Xi, Thermo Fisher) and all the spectra were calibrated with the C 1 s core level spectrum collected at the binding energy of 284.6 eV.
To test the ion–beam current generated by the silver ion source based on the RbAg4I5 superionic conductor, an experimental system consisting of an emitter assembly 1, heating device 2, and ion–beam collector 3 was established, as presented in Fig.
The emitter assembly 1 is composed of a silver tip coated with an RbAg4I5 film by PLD method (ion emitter) and a cylindrical copper (tip holder) in a stainless steel tube. The sliver tip has an apex radius less than 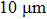
The XRD spectra of the powders obtained by ball-milling of β-AgI and RbI with an agate pestle for 10 min and synthesized after ball milling and subsequent annealing in argon are shown in Fig.
Figure
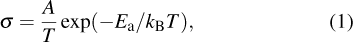 |
The RbAg4I5 film was deposited on the Ag substrate at laser energy of 200 mJ and substrate temperature of 80 °C. The XRD pattern is shown in Fig.
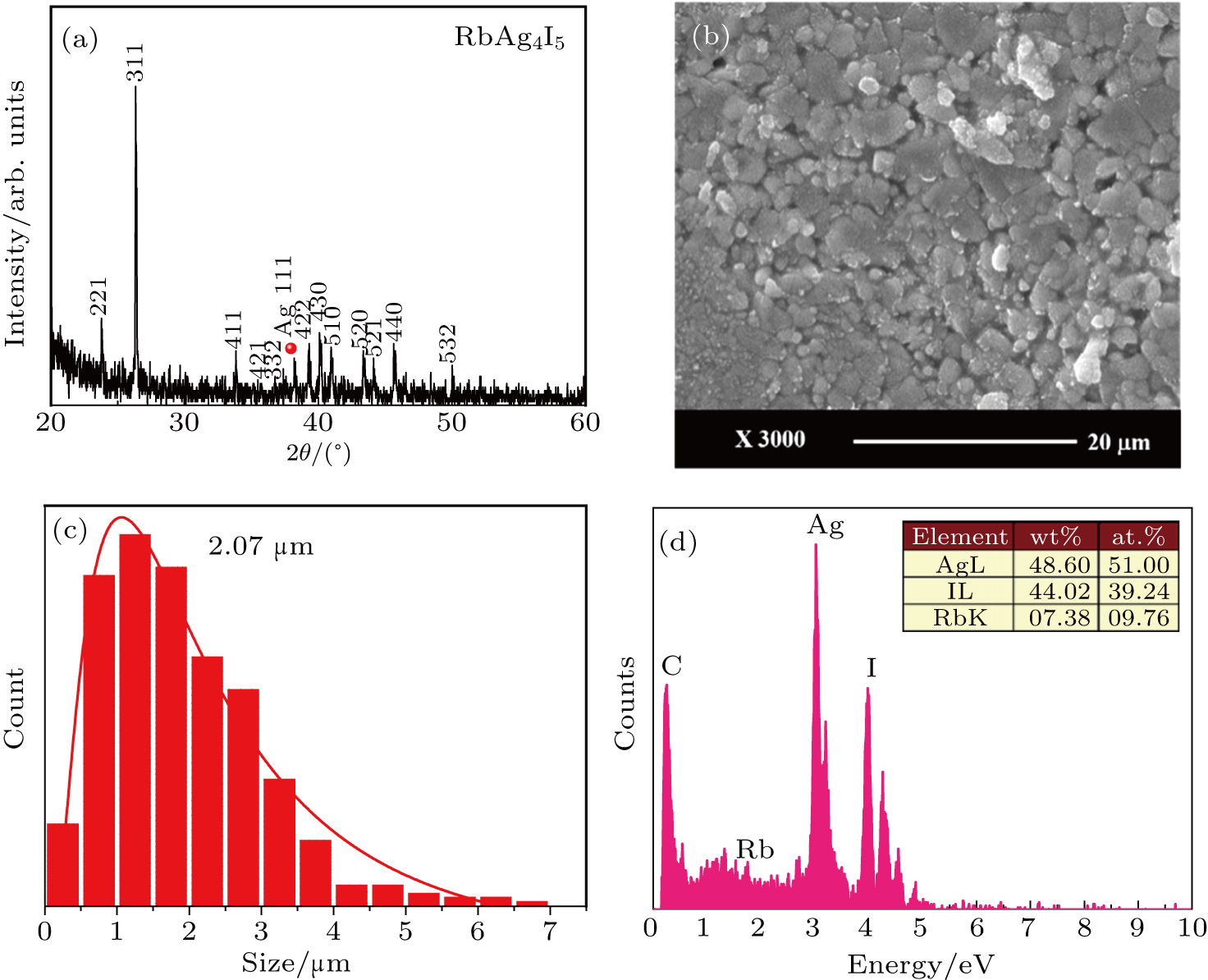 | Fig. 4. Characterization of the RbAg4I5 film: (a) XRD spectrum, (b) SEM image, (c) the particles size distribution, (d) EDS spectrum, the inset shows the elemental composition. |
Figure 
Figure
The elemental composition and chemical bonds in the RbAg4I5 film were measured by XPS. Figure
Figure
 | Fig. 6. Temperature dependence of (a) ion current obtained at 10-kV acceleration potential and (b) conductivity of the RbAg4I5 solid electrolyte film. |
Figure
A running test of the silver ion source under the heating temperature of 196 °C and 10-kV acceleration potential for few minutes was conducted, as shown in Fig.
Mechano-chemical synthesis of the RbAg4I5 powder was performed for the PLD process. The pulsed laser deposited film on Ag substrate at temperature of 80 °C exhibited pure RbAg4I5 phase. The grain structure with the size of 
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] |