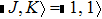† Corresponding author. E-mail:
We demonstrate the production of cold, slow NH3 molecules from a supersonic NH3 molecular beam using our electrostatic Stark decelerator consisting of 179 slowing stages. By using this long Stark decelerator, a supersonic NH3 molecular beam can be easily decelerated to trappable velocities. Here we present two modes for operating the Stark decelerator to slow the supersonic NH3 molecules. The first is the normal mode, where all 179 stages are used to decelerate molecules, and it allows decelerating the NH3 molecular beam from 333 m/s to 18 m/s, with a final temperature of 29.2 mK. The second is the deceleration-bunch mode, which allows us to decelerate the supersonic NH3 beam from 333 m/s to 24 m/s, with a final temperature of 2.9 mK. It is clear that the second mode promises to produce colder (high-energy-resolution) molecular samples than the normal mode. Three-dimensional Monte Carlo simulations are also performed for the experiments and they show a good agreement with the observed results. The deceleration-bunch operation mode presented here can find applications in the fields of cold collisions, high-resolution spectroscopy, and precision measurements.
Inspired by the spectacular achievements in cold atoms, the field of cold molecules has been developed rapidly during the last two decades. It has had a significant impact on modern atomic, molecular, and optical physics, physical chemistry, and fundamental physics,[1,2] and offers new applications and potentials in many fields, such as cold collisions[3–5] and cold chemistry,[6–9] high-resolution spectroscopy,[10,11] precision measurements,[12–14] and so on.
A variety of techniques to produce cold molecules have been developed, including buffer-gas cooling,[15] electrostatic Stark deceleration,[16] Zeeman deceleration,[17,18] optical Stark deceleration,[19] Rydberg–Stark deceleration,[20,21] laser cooling,[22–26] and optoelectrical cooling.[27] The Stark deceleration originates from linear accelerators for charged particles, and achieves full control over the velocity and velocity spread of a polar molecular beam. The first experimental demonstration of Stark deceleration was implemented in 1999, in which a pulse beam of metastable CO molecules was decelerated from 225 m/s to 98 m/s.[16] Since then, a number of molecular species have been decelerated,[28–39] trapped,[20,28,40,41] and employed to important applications.[3,10,11,42] Furthermore, different kinds of decelerators have been designed and constructed, including alternating-gradient (AG) decelerator,[43] chip decelerator,[44] traveling wave decelerator,[45,46] ring Stark decelerator,[47,48] and U-shaped decelerator.[49]
The NH3 molecule is a very promising candidate in molecular physics and physical chemistry and has a variety of applications in the fields of microwave frequency standard, precision measurements, and cold collisions. First, NH3 was employed in the first atomic clock by Condon and Lyons in 1948[50] and the first demonstration of the microwave amplification by stimulated emission of radiation (maser) by Gordon et al. and other groups in 1954–1956,[51–54] and its hyperfine structure in a two-cavity maser was studied in some detail.[55] However, because the central velocities of NH3 molecular beams were fast and their temperatures were high in these experiments, the performance of the NH3 maser was considerably limited. It is clear that if the NH3 molecular beam can be slowed to a very low velocity and cooled to a lower temperature, then the performance of the NH3 maser will be greatly improved. Next, NH3 molecule can be used to realize precision measurements[42] in the molecular beam or fountain experiments, such as testing proton–electron mass ratio (mp/me) and its time-variation,[56] time-reversal symmetry, and study the tunneling inversion, as well as near degeneracies between inversion and rotation energy,[57] and so on. Moreover, the high-energy-resolved cold NH3 molecular beam can also be used to study cold collisions and cold chemistry; for example, measuring the collision cross section and studying its dependence on collision energy.[58,59]
In 2002, the Meijer group first used a traditional Stark decelerator to generate a cold NH3 molecular beam.[29] However, due to the relatively large inversion splitting, it is more difficult to decelerate the NH3 molecule than ND3. To solve this problem, Bethlem group used a complex setup, consisting of a 100-stage traditional Stark decelerator, together with a 336-ring traveling wave decelerator, to slow the NH3 molecular beam and trap the molecules in the traveling wave decelerator in 2013.[41,60] More recently, they were the first to demonstrate a molecular fountain with NH3 molecules by using the previously mentioned two types of Stark decelerators, and a combination of quadrupole lens and bunching elements, and they obtained very cold NH3 molecules.[42] It is well-known that the traveling wave decelerator has many advantages but it is more challenging to implement than the traditional Stark decelerator due to its complex and expensive high-voltage analog amplifiers and electronic controlling system, thus it is difficult to realize traveling wave deceleration for molecules in many laboratories. Consequently, it would be interesting and worthwhile to find a simple experimental setup to realize an efficient deceleration for a supersonic NH3 molecular beam.
In this paper, we implemented the deceleration of NH3 molecules with our 179-stage traditional Stark decelerator to obtain a sufficient cold, slow NH3 packet that can be captured in traps for the applications in cold collisions or high-resolution spectroscopy. The initial supersonic beam of NH3 was slowed down when the decelerator was operated in the normal mode and the deceleration-bunch mode, respectively. Three-dimensional (3D) Monte Carlo (MC) simulations were also performed for the experiments. Several improvements were presented to increase the number of the desired slow molecules for further applications.
Our schematic experimental diagram is shown in Fig. 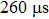
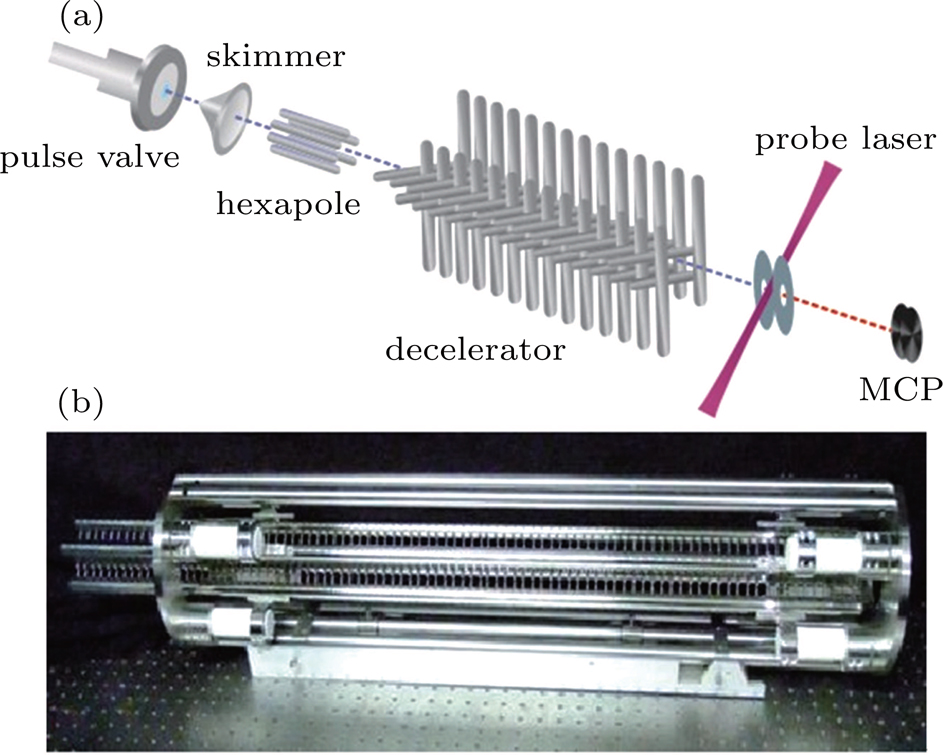 | Fig. 1. (a) Schematic diagram of the experimental setup. (b) Our home-made electrostatic Stark decelerator with 179 deceleration stages. |
After exiting the decelerator, the molecules are state-selectively detected in the area about 46 mm downstream from the last stage of the decelerator by using the (2+1) resonance–enhanced multi-photon ionization (REMPI) scheme with a focused dye laser (PrecisionScan of Sirah Laser und Plasmatechnik GmbH, pumped by pulse Nd: YAG laser of Quanta-Ray PRO-Series) near 322 nm.[42]
The working principle of the Stark decelerator has been discussed in detail elsewhere.[29,61,62] In our experiments presented here, the Stark decelerator is used with different modes of operation, including the guiding mode, the bunching mode, the normal deceleration mode, and the deceleration-bunch mode. The deceleration-bunch mode can be used to improve the energy-resolution of the resulting beam and was firstly demonstrated by Parazzoli et al.[63]
Because of the adiabatic expansion in the process of producing the supersonic beam, most of NH3 molecules in the beam reside in the lowest rotational levels in the vibrational and electronic ground state. To acquire a quantitative understanding of the populations in different levels, we calculate the thermal distribution of the rotational levels of NH3 for different rotational temperatures according to the function[64]
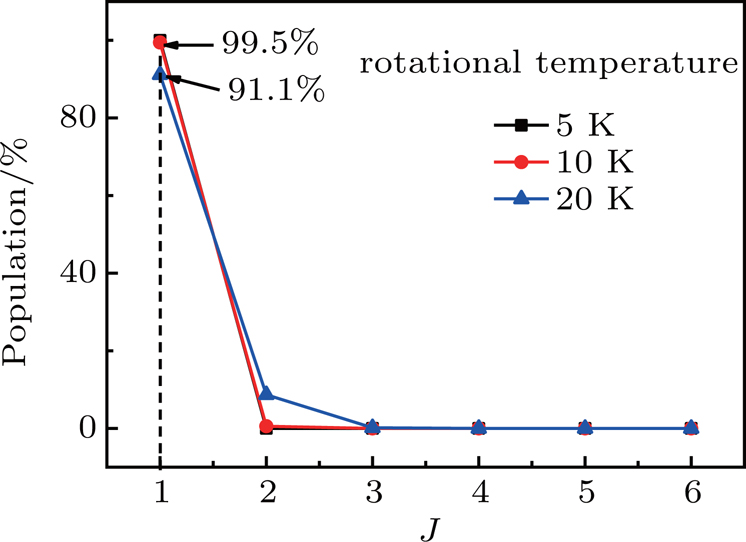 | Fig. 2. The relative populations of the NH3 molecules in different rotational states at the rotational temperatures of T = 5 K, 10 K, and 20 K. |
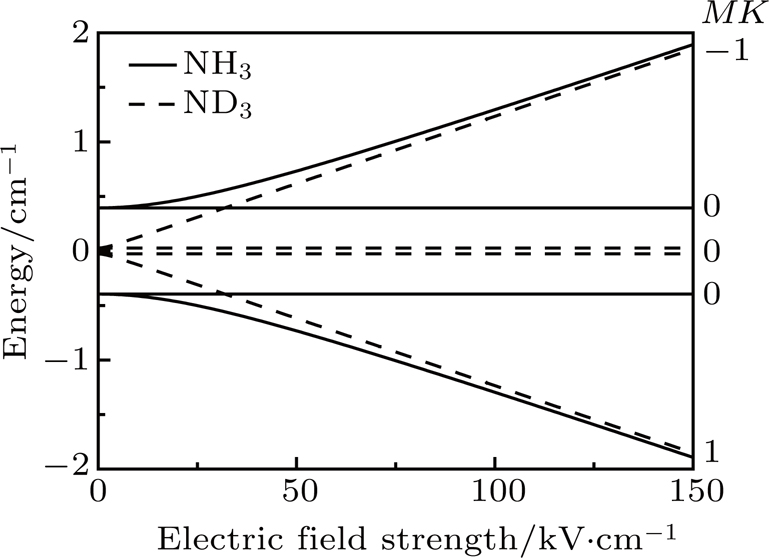
The NH3 molecule in the states 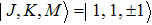
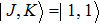
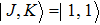
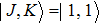
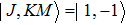
In the experiments presented here, the decelerator is operated at voltages of +10 kV and −10 kV. The guiding mode is used to get the information about the center velocity and the velocity spread of the initial beam for deciding appropriate parameters refer to the dynamic simulations and the deceleration experiments, and the hexapole is applied with zero voltage in this mode.
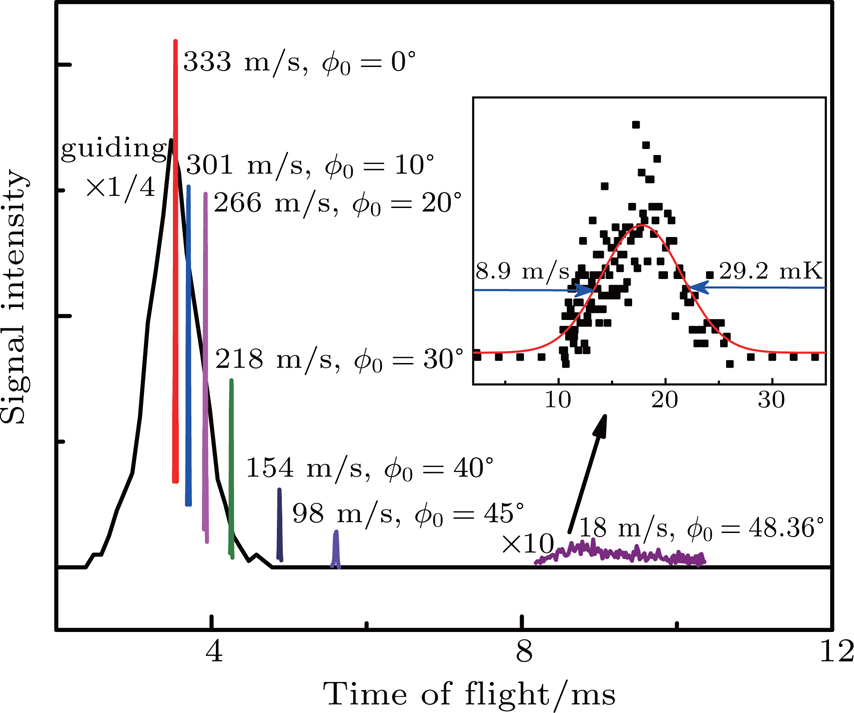
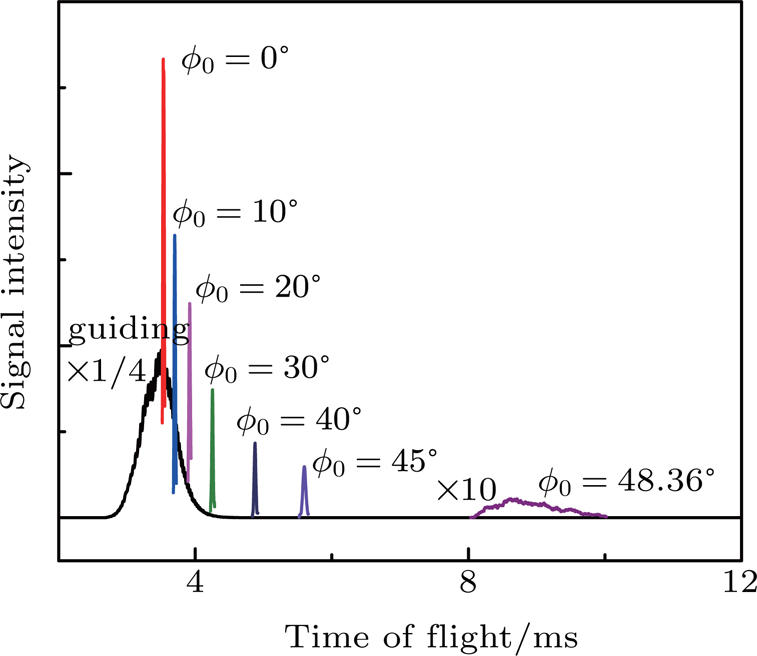
Figure
MC trajectory simulation can help us to understand the behavior of the Stark deceleration process.[29,35,40] The TOF profiles obtained by 3D MC trajectory simulations of the experiments are shown in Fig.
 | Fig. 5. Calculated TOF signals of NH3 by three-dimensional Monte Carlo simulation when the decelerator is operated in the normal mode. |
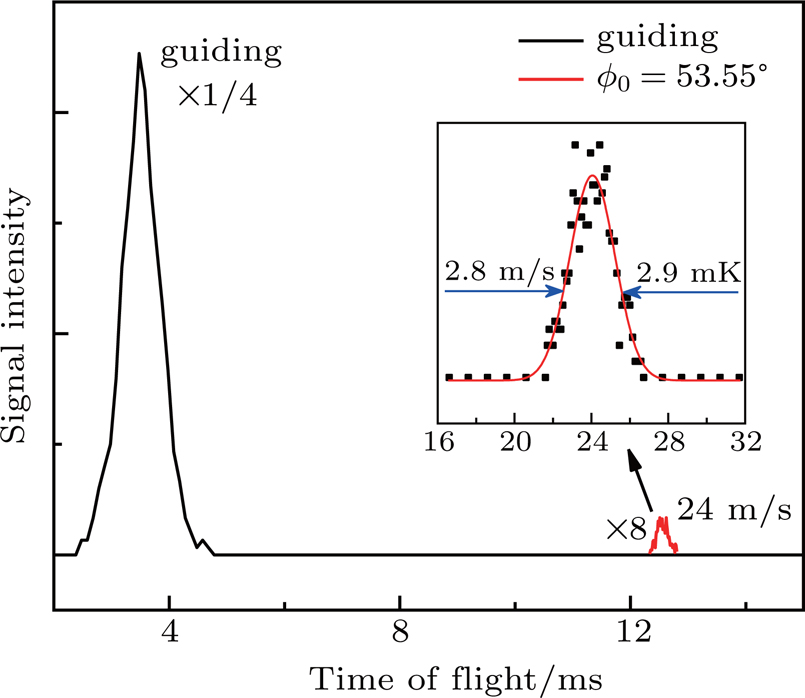
Figure 
We experimentally slowed supersonic beams of NH3 molecules using our 179-stage Stark decelerator in two different operation modes, the normal mode and the deceleration-bunch mode. Starting from an initial speed of 333 m/s, the pulse beam of NH3 molecules seeded in xenon was slowed down to 18 m/s in the normal mode with a synchronous phase angle ϕ0 =48.36°, resulting in a slow packet with a velocity spread of 8.9 m/s, corresponding to a longitudinal temperature of 29.2 mK. Besides, the supersonic beam of NH3 was also decelerated to 24 m/s in the deceleration-bunch mode with a phase angle ϕ0 =53.55° in slowing stages, leading to a colder decelerated packet with a narrower velocity spread of 2.8 m/s, corresponding to a temperature of 2.9 mK, lower than that of the packet obtained in the normal mode by 10 times. 3D MC trajectory simulations have also been performed and the simulated TOF profiles are in good agreement with the observed ones.
These Stark decelerated cold molecules of NH3 can subsequently be trapped in electrostatic traps for some promising applications such as cold collision experiments,[58] high-resolution spectroscopy,[55] and precision measurements.[56,57] Several potential improvements can be made in the experiments. The voltage difference between the electrodes of the decelerator can be increased using the method of high-voltage conditioning with glow discharge of nitrogen and helium, and then the decelerator can be operated at higher voltages in the experiments, leading to more kinetic energy loss per stage. Besides, if the pulse valve is replaced by another one that can be operated down to liquid nitrogen temperatures, the valve can be cooled to a temperature much lower than room temperature such as 200 K, resulting in smaller initial central velocity of the molecular beam, which will also lead to the increase in the number of slow molecules.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] | |
| [62] | |
| [63] | |
| [64] | |
| [65] | |
| [66] |