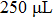† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51877184).
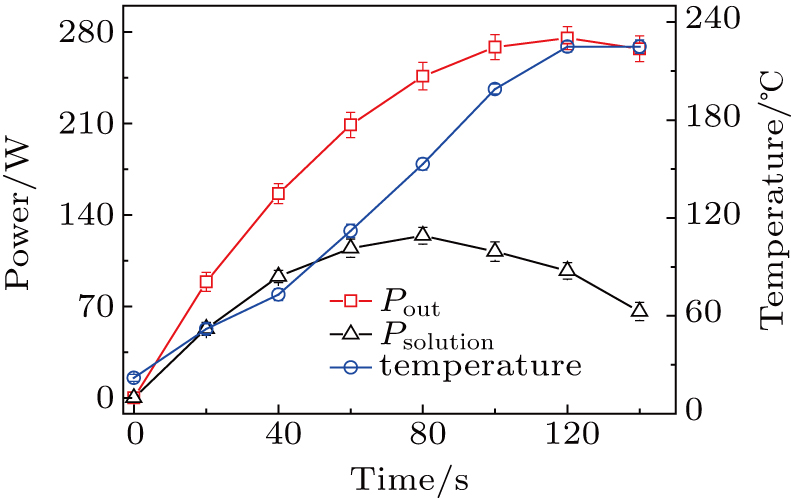
As a renewable carbon resource, biomass can be converted into polyols, aromatic hydrocarbons, alkanes, and other products by traditional catalytic liquefaction method, which has been widely used in production and life. The efficient development and utilization of biomass energy will play a very positive role in solving the problems of energy and ecological environment. A way of combining the plasma electrolysis with traditional catalytic liquefaction realizes the efficient liquefaction of sawdust, which provides a new liquefaction way for traditional biomass conversion. In this experiment, the effects of solution composition, catalyst content and power supply on solution resistance and liquefaction rate are analyzed. It is found that solution composition and catalyst content have a great influence on solution resistance. The results show that the liquefaction rate is highest and the resistance is smallest when the solution resistance is 500 Ω. The liquefaction rate is greatly affected by the solution temperature, and the solution temperature is determined by the output power between the two electrodes. The output power includes the heating power of the electric field and the discharge power of the plasma. We measure the electric potential field distribution in the solution and the plasma power. It is found that the output power between the two poles increases nonlinearly (from 0 to 270 W) with time. In two minutes, the electric field heating power increases from 0 to 105 W and then decreases to 70 W, while the plasma power increases from 0 to 200 W. It is well known that in the first 70 seconds of the experiment the electric field heating is dominant, and then the plasma heating turns into a main thermal source. In this paper, plasma electrolysis and traditional catalytic liquefaction are combined to achieve the efficient liquefaction of sawdust, which provides a new way for biomass liquefaction.
Biomass is defined as the renewable organic material originating from plants including wood wastes, energy crops, aquatic plants, agricultural crops, and their derivatives, as well as the municipal and animal wastes. It is considered as potential renewable sources of fuels and chemical feedstock due to the characteristics of its abundance and neutrality for respective carbon emissions.[1]
The biomass liquefaction is a promising technology for the conversion of biomass into bio-chemicals and liquids bio-fuels. So far, the methods employed for liquefaction mainly include the solvent–catalytic liquefaction,[2,3] hydrothermal liquefaction,[4] and the supercritical/subcritical liquefaction.[5] The applications of hydrothermal liquefaction and the supercritical/subcritical liquefaction are restricted by the strict operation conditions, which normally require high temperature and high pressure to obtain supercritical/subcritical fluid for the post-processing of liquefaction. The solvent-catalytic liquefaction is an effective method to convert biomass into fuels, however this process normally requires unsatisfactorily long time to reach an acceptable yield of bio-fuels.[6,7] For this reason, the development of an alternative process under the mild conditions is very important for the economic viability of biomass liquefaction.
Recently, our research group has combined plasma electrolysis in the presence of sulfuric acid as the catalyst with traditional polyethylene glycol 200 (PEG 200) and glycerol co-solvent liquefaction to achieve a fast liquefaction of pine sawdust.[8] Previous experiments[8] show that the plasma electrolysis liquefaction (PEL) process is also that of solid macromolecules (sawdust) decomposing into small molecules (liquids and gases), which is directly related to the change of solution resistance in the process of PEL. Therefore, it is necessary to carry out further research. Apart from the solution temperature, the power of discharge plasma also directly affects the sawdust liquefaction process. Therefore, it is necessary to analyze the relationship between the power of discharge plasma and liquefaction rate.
Pine sawdust (Fujian) was dried for 12 h in an oven set at 105 °C, then crushed, sieved, and separated using 40–80 meshes. The sifted sawdust was placed in a desiccator at room temperature. The water content, ash content, acid insoluble lignin, cellulose, and alpha cellulous content of the biomass feedstock pine sawdust are summarized in Table
| Table 1.
Compositions of pin sawdust (%). . |
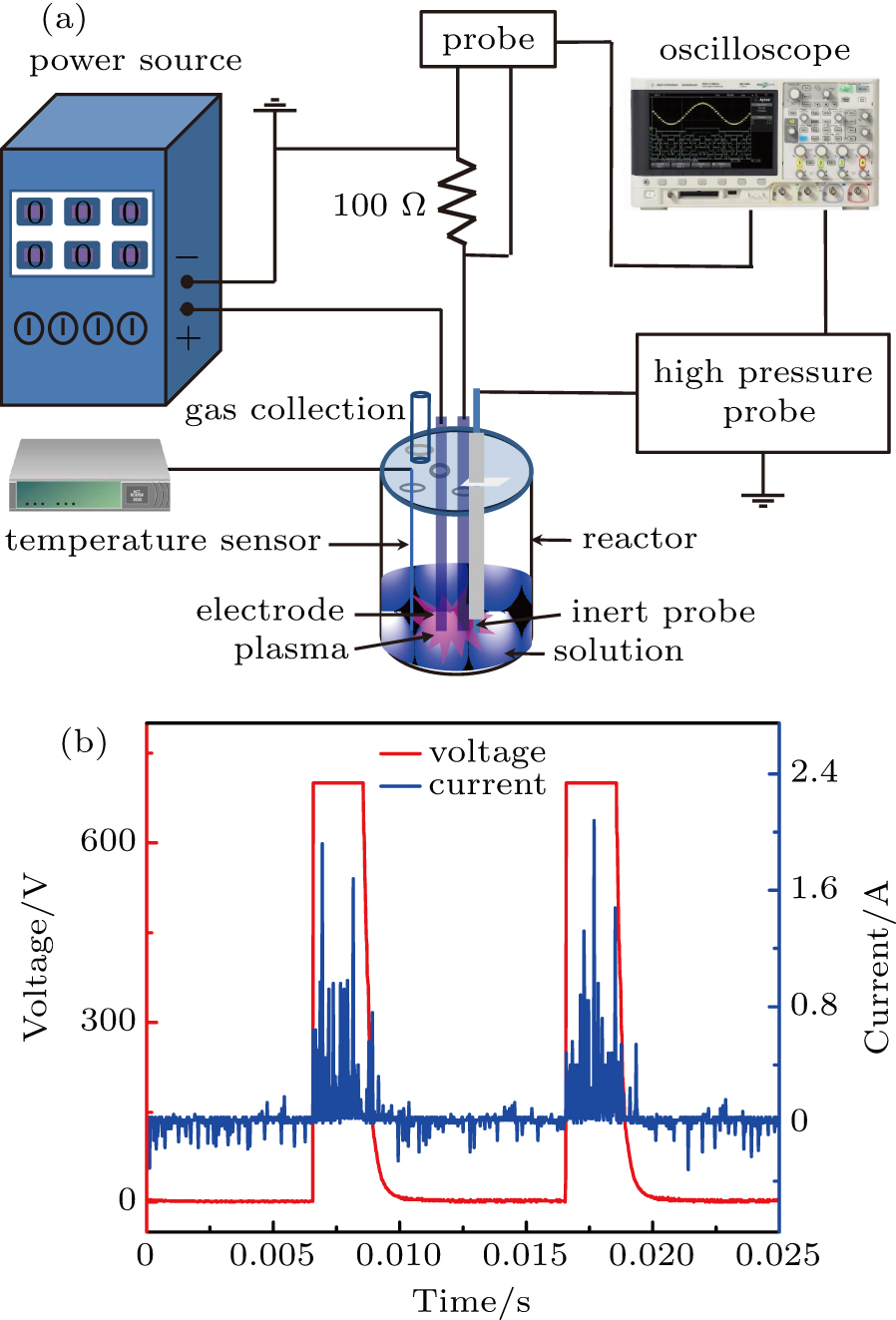
Figure
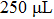
To study the effect of the variation of the solution impedance on the plasma electrolytic liquefaction of sawdust, the copper rods with a purity of 99.9% (diameter, 1.0 mm) were used to replace the tungsten electrodes and connected to the impedance analyzer (Agilent HP4192a) to identify the resistance of the aqueous slurries. Besides, two tungsten wires each with a diameter of 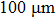
Based on the previous studies of biomass liquefaction,[9,10] the experimental parameters are specified as follows: the amount of sawdust is 5 g, PEG 200 and glycerol are used as co-solvent with a volume of 31 mL and the amount of the catalyst, sulfuric acid was 
In the process of voltage increasing from 0 V to 750 V, a large amount of white smoke is formed on the mixed aqueous slurries and accompanied by a slight explosion. After that the smoke recedes, an arc discharge is clearly observed close to the cathode, the solution near the cathode begins to boil and is followed by the formation of small gas bubbles. Later, the flash becomes larger and brighter, and boiling becomes violent, which is accompanied by splashing of the solution on the inner wall of the reactor and generating the white fog. From the above analyses, the process of sawdust liquefaction can be represented by the plasma electrolysis process,[12,13] thus the input voltage in this case is valued to be 710 V.
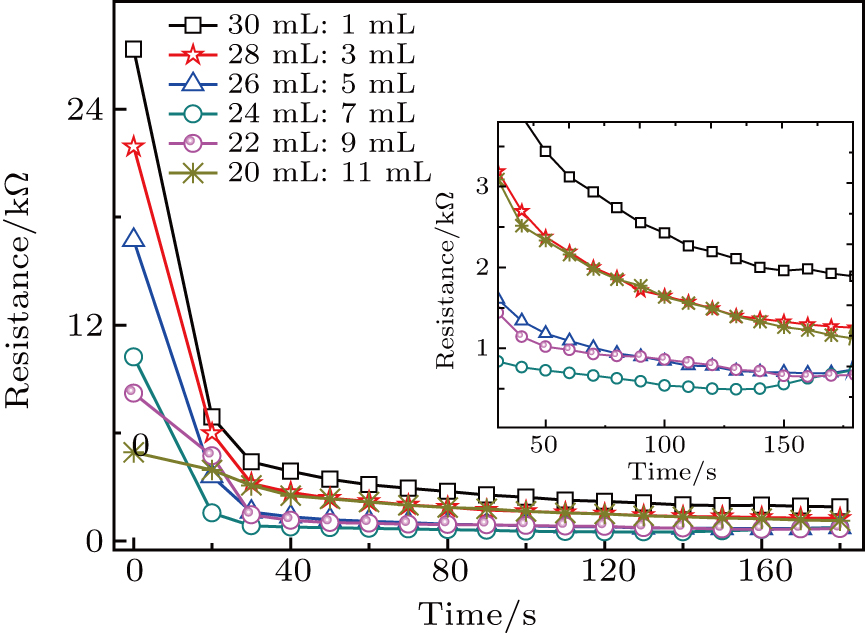
In order to investigate the effect of the liquefaction agent on the resistance in PEL process, the volume ratio of the co-solvent PEG 200 and glycerol is varied from 30:1 to 20:11 (mL:mL) with a total volume of 31 mL unchanged. The input voltage, the 98% sulfuric acid volume, and the biomass feedstock sawdust amount are all kept constant, they are 710 V, 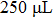
In order to determine how the content of sulfuric acid can affect the solution resistance and the temperature of solution in the PEL process of sawdust, the experiment is conducted under various quantities of catalyst in a range from 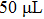



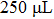
The frequency of power supply used in the plasma electrolysis ranges from 50 Hz to 2 kHz. The experimental results indicate that the change of frequency has no significant influence on the solution resistance. As the homogenized solution used in this research shows the characteristic of pure resistance, no obvious relationship between the frequency and the solution resistance is observed. Therefore, the frequency of power supply is fixed at 200 Hz.
The power duty ratio used in the experiment can be adjusted from 5% to 80%, and the corresponding pulse width changes from 2.5×10−3 s to 40×10−3 s. Since the H+ ionsʼs acceleration time in the solution is proportional to the pulse width, the duty ratio is an essential factor in the PEL process. Figure
The plasma electrolysis technology is used to liquefy the biomass feedstock sawdust. This experiment is conducted at room temperature and atmosphere pressure, where the input voltage is 710 V, frequency and duty ratio of power supply are 200 Hz and 30%, respectively, and the amount of solvent PEG 200, glycerol, and catalyst (sulfuric acid) are 24 ml, 7 ml, and 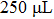
The reason for using 710 V as an input voltage is that the liquefaction process under plasma discharge mainly occurs in the U4 stage, when the cathode is covered by the generated gaseous products and the discharge current can keep steady at 0.2 A with the input voltage increasing from 710 V to 750 V. To analyze the effect of the potential in the solution and radicals generated through the plasma discharge on the PEL process of sawdust, the temperature of oil bath is set to be 225 °C initially followed by heating the aqueous slurries which includes sawdust, PEG 200, glycerol, and catalyst, 98% sulfuric acid. It can be seen in Fig.
 | Fig. 6. Comparison between curves of the liquefaction yield in PEL liquefaction process and conventional liquefaction process. |
In the plasma electrolysis liquefaction process, the increase of the solution temperature is due mainly to its being heated by the potential drops on the solution resistance and the discharge plasma. When the input voltage keeps constant, the potential of the solution will increase with the resistance decreasing, and then, the resistance power can increase according to the following equation:
In order to detect the electrical potential in the solution, two tungsten wires each with a diameter of 
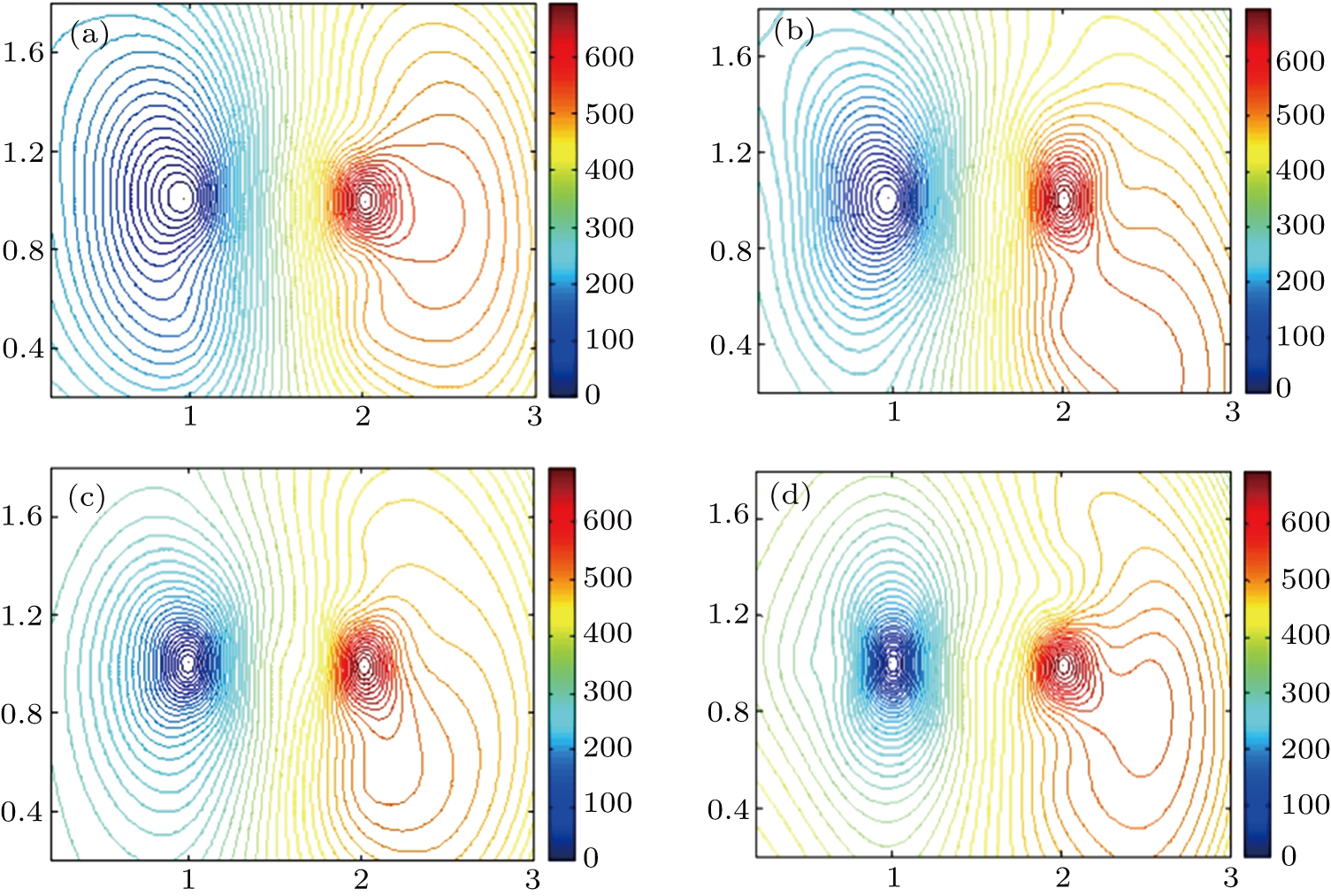 | Fig. 8. Equipotential line distributions for different reaction times: (a) 20 s, (b) 60 s, (c) 120 s, (d) 150 s. |
The H2 and CO were the main gas products from the PEL process. The mole percentage of H2 and CO are 97.7% and 1.8%, respectively. A smaller quantity of hydrocarbons (CH4, C2H6, and C2H4) are also detected with a total mole percentage of 0.5%. This phenomenon indicates that the H2-rich gas is obtained in the PEL process. The components of the gas production from the PEL process are similar to these obtained in the traditional catalytic liquefaction process, but the mole percentage of each component is totally different.[15]
Optical emission diagnostics (OES) is carried out to understand the formation of the reactive species in the PEL process. Figure 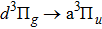

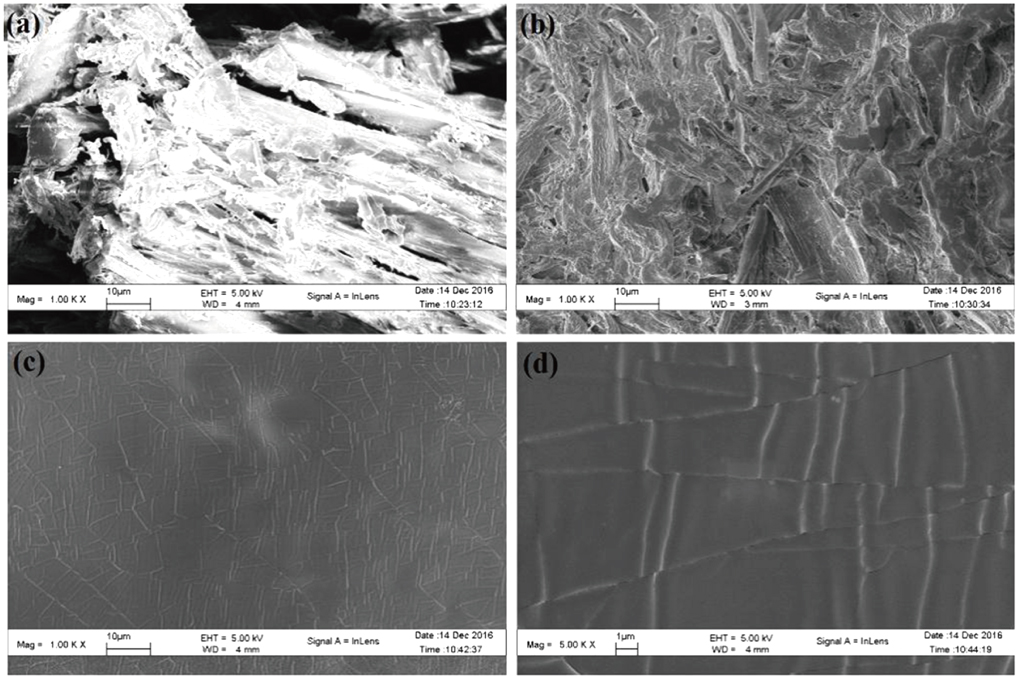
The main components of pine sawdust are lignin, cellulose, and total cellulose, which are mainly composed of polysaccharides. Among these substances, cellulose is a linear chain (glycosidic bond)-linked polysaccharide (molecular weight higher than 5×104) composed of hundreds and thousands of β (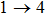
In addition, the infrared spectrum is also used to analyze the chemical properties of the sawdust liquid products and solid residues as shown in Fig.
The liquefied products are complex and difficult to separate, but the content of nitrogen and sulfuric are low. At present, the bio-oil is mainly used for combustion, power generation or biomass adhesives, molded materials, foaming materials, etc.[25,26] For the combustion, the high calorific value of the liquefied product needs to be calculated. The calculation formula is as follows:
| Table 2.
Compositions of liquid product and solid residual (mole ratio%). . |
In the PEL experiment, we find that the solution resistance first decreases and then increases slightly. This process is consistent with that in which cellulose, hemicellulose, and lignin are first decomposed into small molecule compounds, and then polymerized into compounds with larger molecular weight. The results show that the change of solution resistance is consistent with the liquefaction rate, which can be used as a new method to calculate biomass liquefaction. By measuring the resistance heating power and the output power between the two poles, it is found that the electric field heating power is dominant in the first 70 seconds, and then the discharge plasma heating is the main thermal source. At the later stage, the liquefaction rate of biomass is significantly improved. But the initial electric field heating can effectively increase the solution temperature, which is conducive to the generation of discharge plasma. So, the PEL of sawdust is a synergistic process of solution resistance heating and plasma heating. In addition, the liquefaction products in PEL are analyzed. It is found that liquid products mainly consist of aldehydes, ketones, acids and esters, which come from cellulose, hemicellulose, and lignin. Gaseous products produced by discharge plasma mainly consist of H2, CO, CH4, and other gases.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] |