† Corresponding author. E-mail:
Project supported by the Fundamental Research Funds for the Central Universities, China (Grant No. 2018B19414), the Natural Science Foundation of Jiangsu Province, China (Grant No. BK20161501), the Six Talent Peaks Project in Jiangsu Province, China (Grant No. 2015-XCL-010), the National Natural Science Foundation of China (Grant Nos. 51776094 and 51406075), the Program of Henan Provincial Department of Education, China (Grant No. 16A330004), the Special Fund of Nanyang Normal University, China (Grant No. ZX2016003), the Science and Technology Program of Henan Department of Science and Technology, China (Grant No. 182102310609), and the Scientific Research and Service Platform Fund of Henan Province, China (Grant No. 2016151).
Membrane technology has been used for H2 purification. In this paper, the systematic density functional simulations are conducted to study the separation of H2 from the impurity gases (H2, N2, H2O, CO, Cl2, and CH4) by the bilayer porous graphitic carbon nitride(g-C3N4) membrane. Theoretically, the bilayer g-C3N4 membrane with a diameter of about 3.25 Å should be a perfect candidate for H2 purification from these mixed gases, which is verified by the high selectivity (S) for H2 over other kinds of gases (3.43×1028 for H2/N2; 1.40×1028 for H2/H2O; 1.60×1026 for H2/CO; 4.30×1014 for H2/Cl2; 2.50×1055 for H2/CH4), and the permeance (P) of H2 (
H2 is an environmentally-friendly, sustainable and high-density-resource energy.[1] It is regarded as one of the most probable substitutes of fossil fuels to deal with the shortage of energy in the future. With the fast development of science and technology, large-scale H2 production is probable on a long timescale. However, an important problem in the H2 production is the gas mixture.[2] For example, the mixture of H2, H2O, CO, and CH4 gases exists in the H2 production course by the current steam-methane reforming method.[3] The H2 and Cl2 mixed gases come from electrolysis saturated salt water in the chlor alkali industry.[4] Ammonia waste gas produces H2 and N2 mixed gases.[5] Therefore, effectively separating H2 from these mixed gases (H2, N2, H2O, CO, Cl2, and CH4) should be very important for environmental protection and energy development.
There are three kinds of traditional gas separation methods, that is: pressure swing adsorption, cryogenic distillation and membrane separation.[6] The membrane separation method uses a two-dimensional (2D) nanomaterial with single-atom thickness to separate mixed gases, which has the advantages of low consumption and facile operation at room temperatures.[7,8] So far, these kinds of membranes have been reported to separate mixed gases, such as zeolite,[9] silica,[10] and carbon-based polymers,[11–14] and so on.
Generally, a suitable H2 purification membrane should have three characteristics. (i) No/weak interaction between H2 and membrane, which is reflected by small adsorption energy (E ad). The physisorption by the van der Waals (vdW) interaction in the whole process[15] should maintain E ad smaller than 0.3 eV. (ii) There should be a big difference in diffusivity between H2 and other kinds of gases reflected by the diffusion energy barrier (E b) to differentiate the migration velocity between H2 and other kinds of gases. For example, the E b of H2 through the monolayer porous graphene (PG) is 0.37 eV, but that of O2, CO2, and N2 are all larger than 1.20 eV.[16] The difference between E b values of H2 and other gases (N2, CO, CO2, and CH4) is more than 0.8 eV through the divacancy defected 2D silicone,[17] and that between E b values of H2 and CH4 is 0.62 eV through graphdiyne.[18] (iii) Permeance (P) and selectivity (S) are two important indicators to measure the H2 purification effect for membrane.[19] As reported, the S values of H2/CH4 are 1022, 108, 1023 for silicone[20] and some graphene-based membranes, respectively.[18]
In recent years, since the successful experimental isolation of graphene,[21,22] it has been used for gas separation. However, the small pore of 2.80 Å for graphene is smaller than that of the H2 molecule (2.90 Å)[23,24] and thus induces more dense carbon atoms and restricts its application in H2 purification.[24,25] To be effectively used for gas separation, graphene with differently-sized pores has been designed.[25,26] These pores are created by the electron beam punching method[27] or chemical etching method.[28–30] For example, Zhu et al.[31] theoretically predicted the H2 separation performance of a new two-dimensional sp2 carbon allotropes-fused pentagon network. Du et al.[32] explored whether the number of H2 molecules permeating through the porous graphene (PG) increases as the pore size increases. However, when the pore size is much larger than the bond length of the H2 molecule, more N2 molecules will pass through the membrane, which will influence the selectivity of H2/N2. Therefore, too small holes and too large holes in the gas separation membrane will greatly reduce the P and reduce the S, respectively.
A potential membrane for H2 purification should have a suitable hole that can effectively facilitate the separation of H2 from mixed gases.[33] It has been reported that the effective method to obtain suitable pores is the N doping. For example, Zhu et al.[34] have proven the good performance of a newly synthesized porous C2N monolayer for separating He, which had sufficient stability, high S and good P. Lu et al.[35] found that the N doping of carbon nanomaterials could reduce the overlap of the electron density around the pores, which allows the gases to easily pass through the pore. Huang et al.[36] explored whether the substitution of N for C in the PG could reduce the E
b values of the gases. As is well known, the new 2D graphitic carbon nitride (g-C3N4) membrane has been discovered in experiment[37] and is stable.[35–38] Its pore diameter (4.80 Å) is much larger than that of graphene (2.84 Å). Although the S by the monolayer g-C3N4 for H2/CH4 and H2/CO (1054 and 1023 at 300 K)[39] is bigger than that for nitrogen doped graphdiyne[40] (H2/CH4 is 1012 and H2/CO is 105 at 300 K) and PG[17] (H2/CO is 1021 at 300 K). However, the P of H2 through the monolayer g-C3N4 membrane at 300 K (18.5 mol/m2sPa calculated by us) is much smaller than that for nitrogen doped graphdiyne (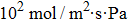

There is an interesting Robeson upper bound phenomenon[44] of the membrane. The highest S at a given P lay near or on a line is called the upper bound.[45,46] Higher P tends to have lower S, but larger holes often induce higher P.[45,46] Therefore, the Robeson upper bound has the P–S trade-off. Thus, effectively reducing the pore size in the monolayer g-C3N4 membrane to further improve the S and P of H2 from mixed gases (H2, N2, H2O, CO, Cl2, and CH4) should be an urgent task.
Moreover, it has been discovered that the pore diameter can be adjusted by constructing a bilayer membrane because the upper and lower layer can be shifted or rotated, which can effectively reduce the pore size and improve the P and S of the H2 from mixed gases because of the boundary overlapping.[36,47,48] For example, Bartolomei et al.[49] have confirmed that the three-layer graphene with an interlayer displacement of 1.6 Å can narrow the size of the hole, which can make it convenient for the H2 and N2 molecules to penetrate. Huang et al.[36] built a quasi-2D new carbon allotrope, which is formed by the bilayer PG through the interlayer bonding and can simultaneously improve the P of H2 and S of H2/CH4.
A review of the literature reveals that there has been no study of H2 purification from mixed gases (H2, N2, H2O, CO, Cl2, and CH4) with a bilayer g-C3N4 membrane. However, monolayer g-C3N4 has been successfully isolated experimentally.[50,51] Moreover, the bilayer 2D membrane can be easily obtained, such as bilayer graphynes,[49] bilayer polyphenylene,[52] bilayer graphene,[53] etc. Therefore, the bilayer g-C3N4 membrane can be easily obtained experimentally. Theoretically, the effective pore size of the bilayer g-C3N4 membrane with no interlayer shift in the horizontal direction is about 3.25 Å, which is a little larger than that for H2 molecule(2.90 Å) and much smaller than that for other gas molecules (3.24 Å for H2O, 3.64 Å for N2, 3.46 Å for O2, 3.74 Å for CO, 3.80 Å for CH4, 3.14 Å for Cl2). Therefore, the bilayer g-C3N4 membrane should be ideal for H2 separation from impurity gases (H2, N2, H2O, CO, Cl2, and CH4). In this paper, we will first describe the computational details in section
All of our structure and electronic calculations are carried out by the density functional theory (DFT) implemented in the DMol3 code.[54] The Perdew–Burke–Ernzerhof (PBE)[55] functional of the generalized gradient approximation (GGA)[56] is employed for the exchange correlation potential.[57] The double numerical basis sets with the polarization functionals basis sets (DNP) are used. The approximate semi-classical dispersion correction scheme DFT+D proposed by Grimme (PBE+D2) is employed in all the calculations to consider the van der Waals forces.[36,58,59] The vacuum space of 20 Å is set in a 2×2×1 supercell, which is large enough to avoid the interaction of periodic image. During the geometric optimization, the convergence criteria are 2×10−5 Hartree (1 Hartree=4.3597×10−18 J) for energy, 4×10−3 Hartree/Å for Hellmann–Feynman force, and 5×10−3 Å for atomic displacement. The criterion of self-consistent field energy is 1×10−5 Hartree.
The accuracy of our method is first tested for the monolayer g-C3N4 membrane. The optimized average bond length of C–N is 1.34 Å, which is in good agreement with the value of 1.41 Å presented by other researchers.[18,36,39] The calculated energy gap (E g) between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is 1.28 eV, which close to that of the condensed graphitic carbon nitride obtained from its ultraviolet-visible spectrum by Zhang et al.[51] Above all, the computational scheme used in this paper is reliable.
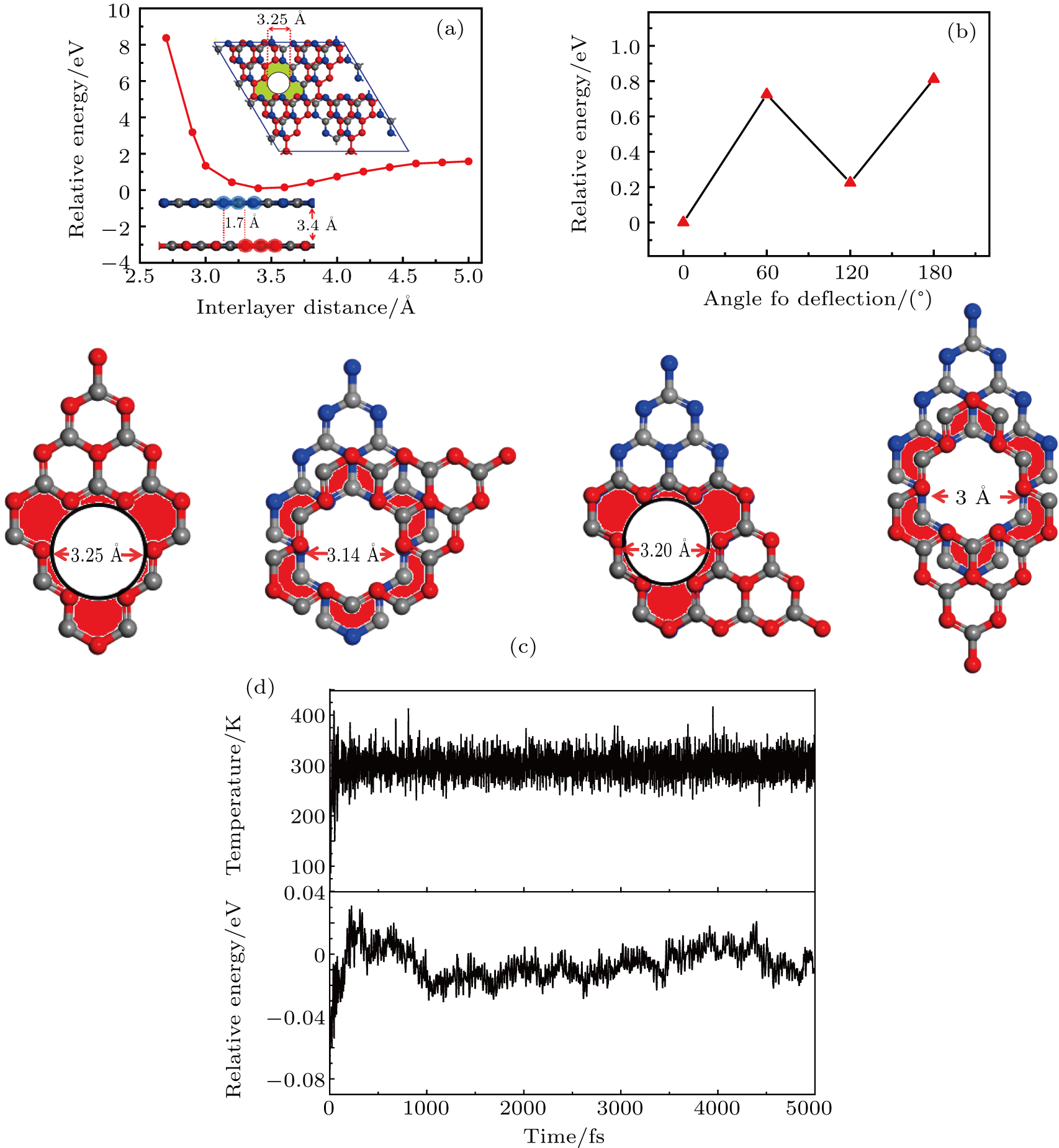
We first find out the most stable geometric structure of the bilayer g-C3N4 membrane. The 2 × 2 supercell is shown in Fig.
Importantly, because of the overlap of the boundary, the effective diameter of the pore decreases from the ∼4.8 Å of the monolayer g-C3N4 membrane to about ∼3.25 Å of the bilayer g-C3N4 membrane, which is larger than that for H2 molecule (2.90 Å) and smaller than that for other gas molecules (3.24 Å for H2O, 3.64 Å for N2, 3.46 Å for O2, 3.74 Å for CO, 3.14 Å for Cl2, 3.80 Å for CH4). Therefore, the bilayer g-C3N4 membrane should be ideal for H2 separation from impurity gases (H2, N2, H2O, CO, Cl2, and CH4).
To contain the hole in the up and down layer in the bilayer g-C3N4 membrane, which are aligned (0°), and confirm the most stable bilayer g-C3N4 membrane, the upper layer is rotated 60°, 120°, and 180°clockwise, resulting in three different bilayer g-C3N4 membranes; as shown in Fig.
The most stable adsorption position of single gas molecule on the bilayer g-C3N4 membrane is discussed in the following. For the various adsorbed gases, the equilibrium adsorption height (D
ad) values between the adsorbed gases and the membrane, and also the adsorption energy (E
ad) values of the molecules on the membrane are listed in table
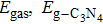

| Table 1.
Values of D ad, E ad and E b of H2, N2, H2O, O2, CO, Cl2, and CH4 gas molecules, and values of S of H2 over other gases and values of P when gases passing through membrane at 300 K. . |
To further examine the stability of the bilayer g-C3N4 membrane, the molecular dynamics simulation with a Nose-Hoover thermostat at 300 K is performed for the 2 × 2 supercell of the bilayer g-C3N4 membrane for 5 ps. The 5 ps has proved sufficient to simulate the whole dynamic course.[62–64] The curve of energy versus time at 300 K is shown in Fig.
The Eb for the penetration of the gas molecule from the pore of the bilayer g-C3N4 membrane listed in table
 | Fig. 2. Equilibrium adsorption configuration of H2, N2, H2O, CO, CO2, and CH4 (top view and side view). The red molecules are different gas molecules. |
Generally, if the E
b is smaller than 0.2 eV, then the penetration process can be realized at room temperature without additional pressure. In addition, the Eb is an important parameter for calculating S and P. It can found from table
The S of the bilayer g-C3N4 membrane can indicate the purity of H2 after separating H2 from mixed gases. The S can be calculated from the Arrhenius equation[17,18] as follows:


 | Fig. 4. Plots of (a) selectivity and (b) permeance versus temperature for H2, O2, N2, Cl2, CO, CH4, H2O molecules passing through the bilayer g-C3N4. |
The performance of a membrane lies on its P, which is defined as 









The charge density isosurfaces of the transition state structure for seven kinds of gas molecule penetration through the bilayer g-C3N4 membrane are shown in Figs.
To explore the purification process of H2 from the mixed gas at room temperature, the molecular dynamics simulation under the realistic condition is performed to reproduce the permeating process of mixed gases to pass through the bilayer g-C3N4 membrane. The initial pressure is set to be 50 atm (1 atm = 1.01325×105 Pa) and the temperature is set to be 300 K. At the beginning of the MD simulation, the gas molecules are placed between two bilayer (5 × 5) g-C3N4 wall, and 120 gas molecules (20 H2 molecules and 20 CH4 molecules, 20 N2 molecules, 20 Cl2 molecules, 20 CO molecules, 20 H2O molecules) are located on the left side of the wall. The simulated total time is set to be 5000 ps. The snapshots of mixture of 120 gas molecules at 0 ps, 500 ps, 1000 ps, and 5000 ps are shown in Fig.
 | Fig. 6. Snapshot of mixture permeating through (a) bilayer g-C3N4 membrane and (b) monolayer g-C3N4 membrane in the simulation for 0 ps, 500 ps, 1000 ps, and 5000 ps. White balls denote H2 molecules. |
The molecular dynamics simulation reveals that 11 and 13 H2 molecules are on the right-hand side of the bilayer g-C3N4 membrane after 1000 ps and 5000 ps at 300 K, while 13 H2 molecules, 2 H2O molecules, 1 N2 molecule and 1 CO molecule permeate the monolayer g-C3N4 after 1000 ps, 13 H2 molecules, 2 H2O molecules, 3 N2 molecule, 3 CO molecule, and 4 CH4 permeate the monolayer g-C3N4 after 5000 ps at 300 K as shown in Fig.
The bilayer porous graphitic carbon nitride (g-C3N4) membrane has suitable pores with a diameter of about 3.25 Å for H2 purification from the impurity gases (H2, N2, H2O, CO, Cl2, and CH4). The average adsorption energy reveals that most gas molecules except for H2O are physically adsorbed on the bilayer g-C3N4 membrane. Compared with the monolayer g-C3N4 membrane, the bilayer g-C3N4 membrane can effectively reduce the diffusion energy barrier of H2, which is of benefit to H2 purification. The bilayer g-C3N4 membrane has high selectivity for H2 over other kinds of gases (H2/N2 is 3.43×1028; H2/H2O is 1.40×1028; H2/CO is 1.60×1026; H2/Cl2 is 4.30×1014; H2/CH4 is 2.50×1055) and the permeance of H2 (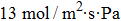
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] | |
| [62] | |
| [63] | |
| [64] |