† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 51602021 and 51474017) and the Fundamental Research Funds for the Central Universities (Grant No. FRF-TP-15-107A1).
ZnO/graphene/polyaniline (PANI) composite is synthesized and used for photoelectrocatalytic oxidation of methane under simulated sun light illumination with ambient conditions. The photoelectrochemical (PEC) performance of pure ZnO, ZnO/graphene, ZnO/PANI, and ZnO/graphene/PANI photoanodes is investigated by cyclic voltammetry (CV), chronoamerometry (J–t) and electrochemical impedance spectroscopy (EIS). The yields of methane oxidation products, mainly methanol (CH3OH) and formic acid (HCOOH), catalysed by the synthesized ZnO/graphene/PANI composite are 2.76 and 3.20 times those of pure ZnO, respectively. The mechanism of the photoelectrocatalytic process converting methane into methanol and formic acid is proposed on the basis of the experimental results. The enhanced photoelectrocatalytic activity of the ZnO/graphene/PANI composite can be attributed to the fact that graphene can efficiently transfer photo-generated electrons from the inner region to the surface reaction to form free radicals due to its superior electrical conductivity as an inter-media layer. Meanwhile, the introduction of PANI promotes solar energy harvesting by extending the visible light absorption and enhances charge separation efficiency due to its conducting polymer characteristics. In addition, the PANI can create a favorable π-conjunction structure together with graphene layers, which can achieve a more effective charge separation. This research demonstrates that the fabricated ZnO/graphene/PANI composite promises to implement the visible-light photoelectrocatalytic methane oxidation.
Methane, as the principal constituent of natural gas, has long been considered to be a preferable energy alternative to traditional fossil fuels due to its high hydrogen-to-carbon ratio and lower CO2 emissions. The global methane reserve is estimated to be approximately 1.4×1011 m3.[1] However, the low energy density of methane compared with those of traditional fossil fuels restrains its on-board applicability, and makes transportation and storage difficult. It is also notable that methane is the second largest greenhouse gas that causes global warming as a result of the greenhouse effect.[2–4] Over the past two hundred years, thanks to the rapid changes in human production, a large amount of methane has been released into the atmosphere. The damaging effect of methane on the ozone layer is 20 times that of carbon dioxide. Therefore a mild method of catalytic conversion of methane into methanol and other oxygenates is of considerable practical value. In this way, the hydrocarbon of methane conversion may be used directly as fuel or may be converted into other oxygenated fuels and valuable products.
In earlier decades, much effort has been made to convert methane into fuels via thermo-catalysis.[5–7] However, the reaction process always requires high temperature or high pressure, which causes great energy consumption. Thus, other methods have been explored to achieve methane oxidation, such as electrocatalytic oxidation of methane. Until now, metal electrodes such as Pt, Au, Pd, and Ru, etc. have been investigated for methane oxidation and different products have been obtained such as CO and CO2.[8–11] Nonetheless, these systems require either uneconomical noble metal or specific electrolyte (e.g., strong acid or alkaline). Being a mild reaction condition and environmentally friendly technique, photocatalysis is a viable technology for methane oxidation because it has shown promising utilization in many fields such as air purification and water splitting.[12–14] For example, WO3, TiO2, and NiO were reported as photocatalysts in methane oxidation process under the irradiation of ultra-violet (UV) or visible laser.[1,15–17] Ag decorated ZnO was also used as photocatalyst to oxidize methane under simulated sunlight illumination.[18] V-containing MCM-41 catalyst was synthesized and its photoactivity was evaluated for the selective photocatalytic oxidation of methane with NO under UV irradiation.[19] However, photocatalytic oxidation has several drawbacks, such as the high photoinduced charge recombination rate and low charge separation efficiency, which hinders the application of semiconductor photocatalysts. An attractive strategy to increase the photocatalytic efficiency is to apply an external bias potential to the anode coated by the photocatalyst, which is known as photoelectrocatalysis.[20–22]
One-dimensional ZnO nanomaterials are wide bandgap semiconductor materials with significant advantages in electrical transport, optics, optoelectronics, photocatalysis, field emission, electrochemistry, etc.[23–26] In addition, one-dimensional ZnO nanomaterials have high electron mobility, exceptional photoconductivity characteristics, large specific surface area, stable chemical properties, abundant sources, and low cost, which makes them an ideal material for preparing the photoelectrocatalysis.[27,28] However, the low utilization rate of visible light due to its wide bandgap and high recombination rate of photogenerated carriers limits the application of ZnO in the field of photocatalysis. Therefore, many studies have been attempted to improve the photocatalytic activity of ZnO, such as doping,[29–31] deposition of quantum dots,[32,33] and construction of heterojunctions with other semiconductors.[34,35] In particular, coupling ZnO with carbon nanomaterials, such as graphene to form a carbon–ZnO photocatalyst, has proven to effectively promote the photocatalytic efficiency. The extremely high electron mobility and large specific surface area of graphene enables it to act as an electron mediator to inhibit photoinduced charge carriers from being recombined.[36–38]
The process of photoelectrocatalytic oxidation can enhance the quantum efficiency by introducing an external bias potential. With regard to this process, on the one hand, PANI is one of the conducting polymers with the delocalized conjugated structure, and its benzenoid and quinonoid units with several redox states make it a promising candidate in electrocatalysis.[39,40] On the other hand, the PANI has high charge carrier mobility and visible-light absorption efficiency, making it an ideal supplier for electron donors and hole transporters upon visible light excitation in photocatalytic process.[41,42]
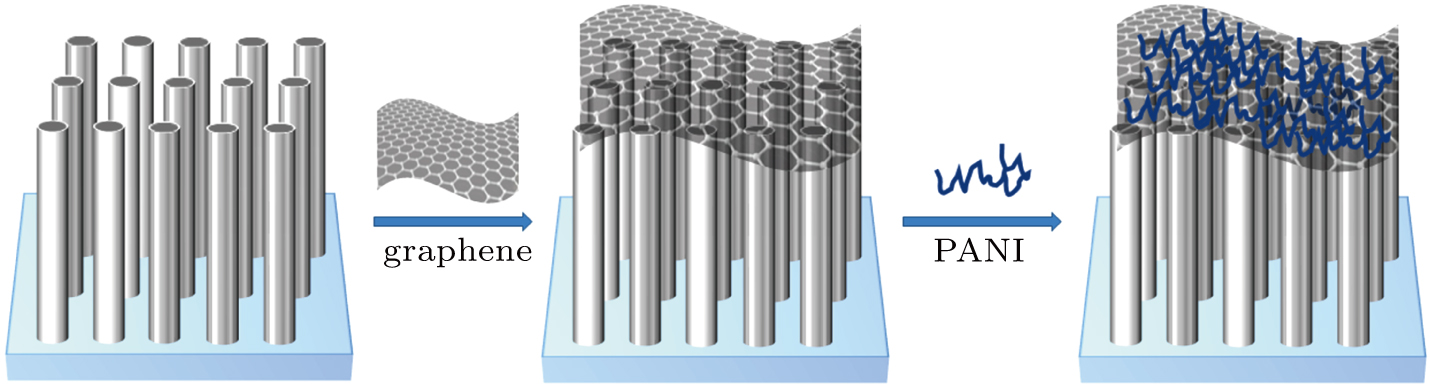
So far, there has been little research on methane oxidation via photoelectrocatalysis. In the present work, for the first time methane is converted into methanol and formic acid via photoelectocatalysis under simulated sun light illumination in ambient conditions. First, we synthesize ZnO NWAs, ZnO/graphene, ZnO/PANI and ZnO/graphene/PANI composites and characterize the morphostructures, crystalline phases and absorption spectra of the samples. Then, their PEC properties are investigated under simulated sunlight illumination by recording the CV, J–t, and EIS curves. The as-prepared ZnO/graphene/PANI nanocatalyst shows a remarkable photocatalytic activity for visible light photoelectrocatalytic methane oxidation. The enhanced performance is attributed to the fact that the introduction of graphene and PANI extends the visible light absorption with enhanced solar energy harvesting and increases the separation efficiency of photo-induced charge carriers by inhibiting the electron–hole pairs from being recombined, thus resulting in an increase in the number of holes that participated in the photo-oxidation process. This research demonstrates that the fabricated ZnO/graphene/PANI composite greatly promises to implement the simulated sun light photoelectrocatalytic methane oxidation. Furthermore, it contributes to a better understanding of the mechanism of the photoelectrocatalytic process converting methane into methanol and formic acid, which brings new ideas to the direct oxidation and utilization of methane.
The ZnO NWAs were prepared by the hydrothermal method.[43] First, the FTO glasses (1 cm×1.5 cm) were cleaned by ultrasonication in acetone, ethanol and deionized water, sequentially for 10 min. Then, the colloidal seed solution (0.25-mM Zn(CH3COO)2·2H2O solution) was spin-coated onto an FTO substrate. After being dried naturally, the FTO glasses were annealed for 30 min at 350 °C. Then they were placed into a polytetrafluoroethylene autoclave containing 150 ml of zinc nitrate hydrate [Zn(NO3)2·6H2O] (25 mM) and hexamethylenetetramine [(CH2)6N4] (25 mM) aqueous solution and were kept at 95 °C for 8 h. Finally, the glasses were cleaned with deionized water carefully to remove the residual surface salts.
Figure 


This coating process was repeated three times to obtain the graphene thin film. The PANI (Macklin Ltd., Shanghai) was dispersed in ethanol to form a 10-
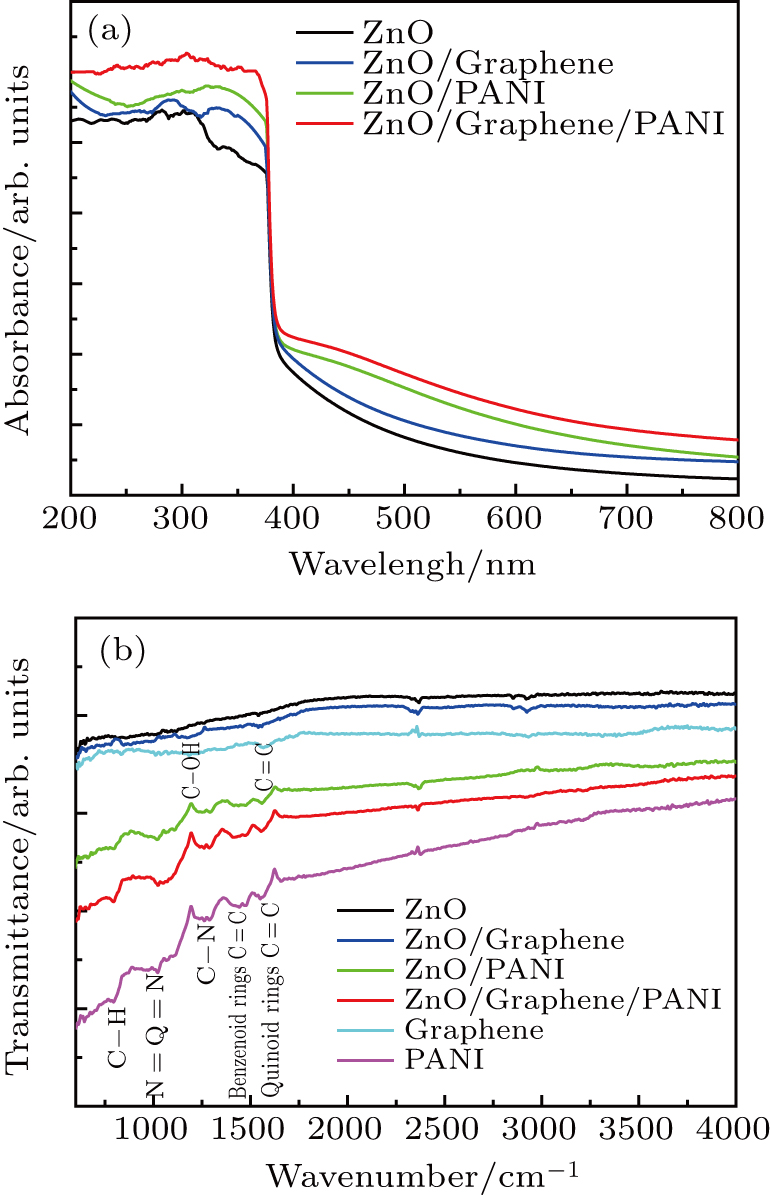
We acquired scanning electron microscopy (SEM) images with a SUPRA55 field-emission scanning electron microscope (FESEM). A Rigaku DMAX-RB x-ray diffractometer, equipped with a Cu Kα x-ray radiation source, was used to examine the x-ray diffraction patterns (XRD) of the samples. Fourier-transform infrared spectra (FT-IR) of the samples are recorded on an Excalibur 3100 spectrometer in a range of 400 cm−1–4000 cm−1. A Cary 5000 UV-vis-NIR spectrophotometer was used to obtain the absorption spectra of the samples over a range of 200 nm–800 nm.
The photoelectrocatalytic oxidation of methane was conducted in a glass electrolytic cell with a 180-ml capacity. Inside the electrolytic cell, a three-electrode system, connected with an electrochemical workstation (CHI 660E, CH Instruments Inc., USA), was used to investigate the PEC properties of ZnO, ZnO/graphene, ZnO/PANI and ZnO/graphene/PANI photoanodes. The three-electrode system contained a saturated Ag/AgCl reference electrode (0.197 V), a Pt counter electrode and a working electrode. The working electrode was FTO glasses coated with ZnO, ZnO/Graphene, ZnO/PANI or ZnO/Graphene/PANI and, sealed by polyacrylate glue to cover the edges and backside. The electrolytic cell was filled with 130-ml Na2SO4 aqueous solution (0.05 M). The photoelectrocatalytic experiments were carried out at room temperature and atmospheric pressure. In a typical reaction batch: after the three-electrode system and electrolyte were prepared ready, argon was injected into the electrolytic cell through the gas intake tube at a flow rate of 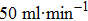
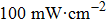

Figure 
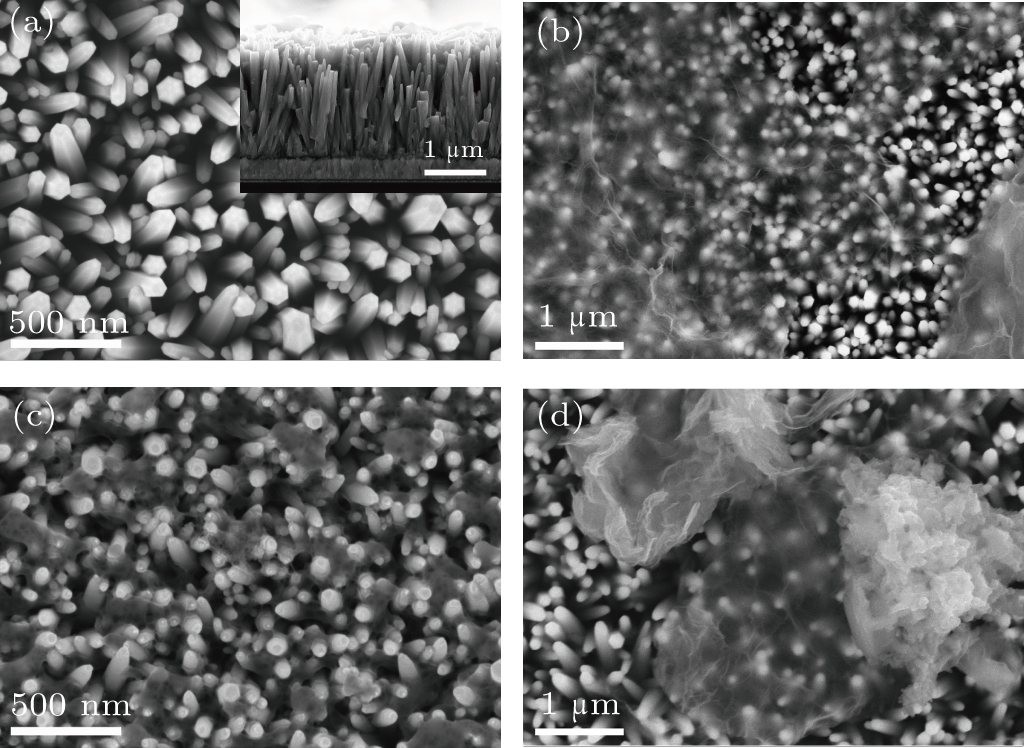 | Fig. 2. Top view SEM image of (a) pristine ZnO NWAs, (b) ZnO/graphene, (c) ZnO/PANI, and (d) ZnO/graphene/PANI composite. The inset shows cross-sectional view of ZnO NWAs. |
Figure
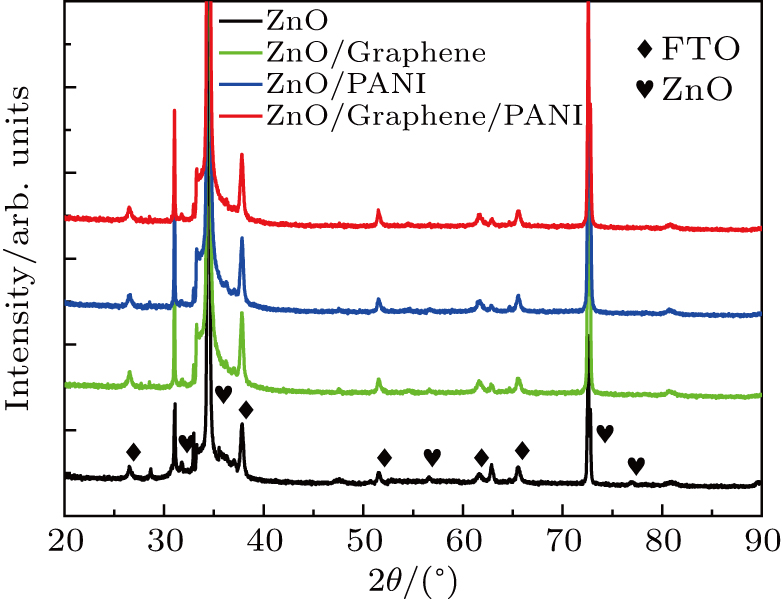 | Fig. 3. XRD pattern of ZnO NWAs, ZnO/graphene, ZnO/PANI, and ZnO/graphene/PANI composite on FTO substrate. |
Figure 
Figure
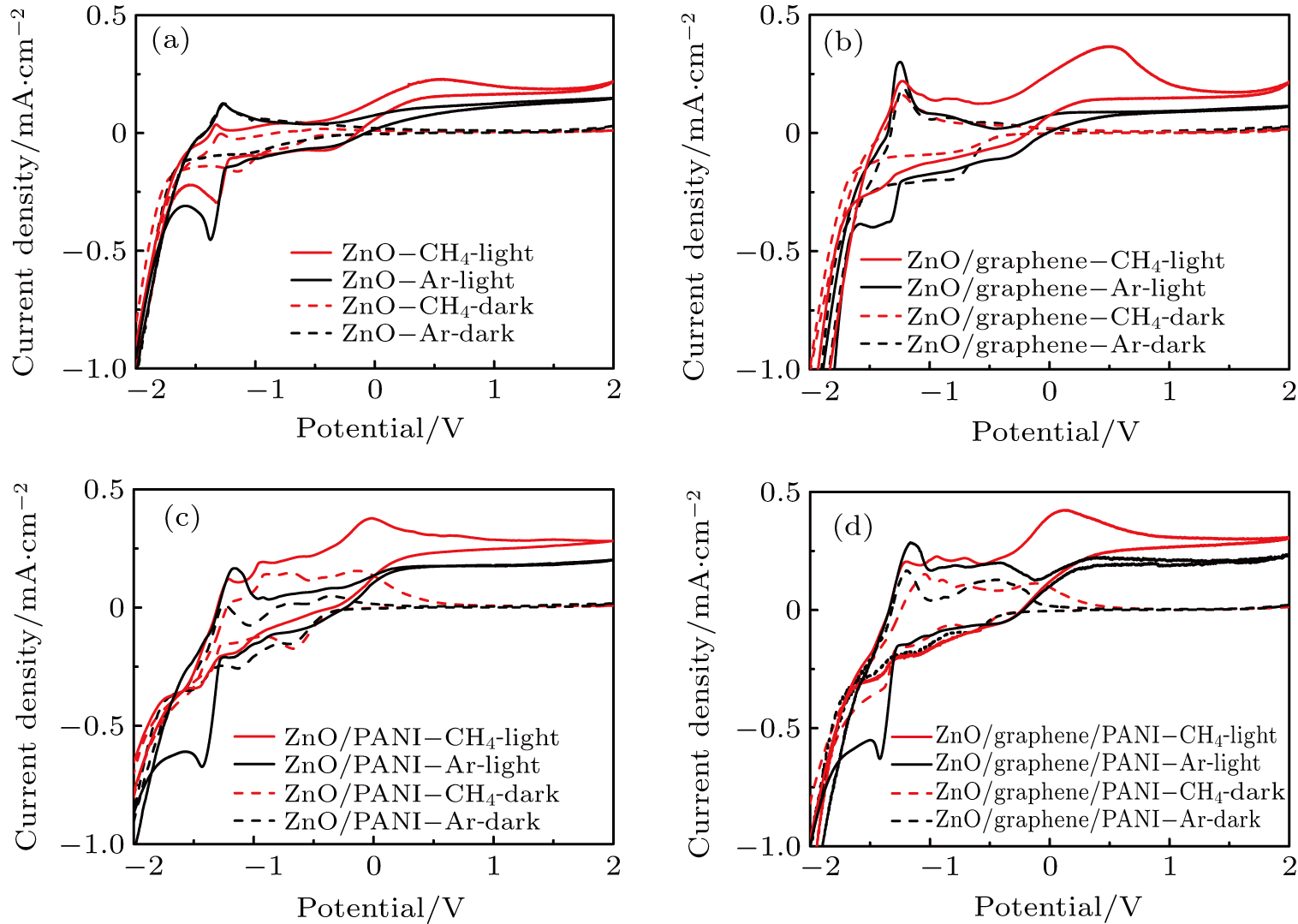
Cyclic voltammograms are recorded in the dark and under illumination to explore the reactions that occur throughout the entire potential window. As can be seen in Fig. 
Figure
In Fig.
Figure 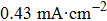
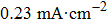
| Table 1.
Comparison among electrocatalytic activities of methane oxidation on ZnO, ZnO/graphene, ZnO/PANI, and ZnO/graphene/PANI. . |
The electrochemistry activity of an electrocatalyst is determined by its intrinsic activity and the number of active sites.[57] Thus, the electrocatalytically active surface area (ECSA) is estimated to reveal the electrochemistry activity of the as-prepared electrodes. The double-layer capacitance (C
dl), which is generally proportional to the ECSA,[58] is investigated by using cyclic voltammetry (CV) as shown in Figs. 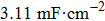
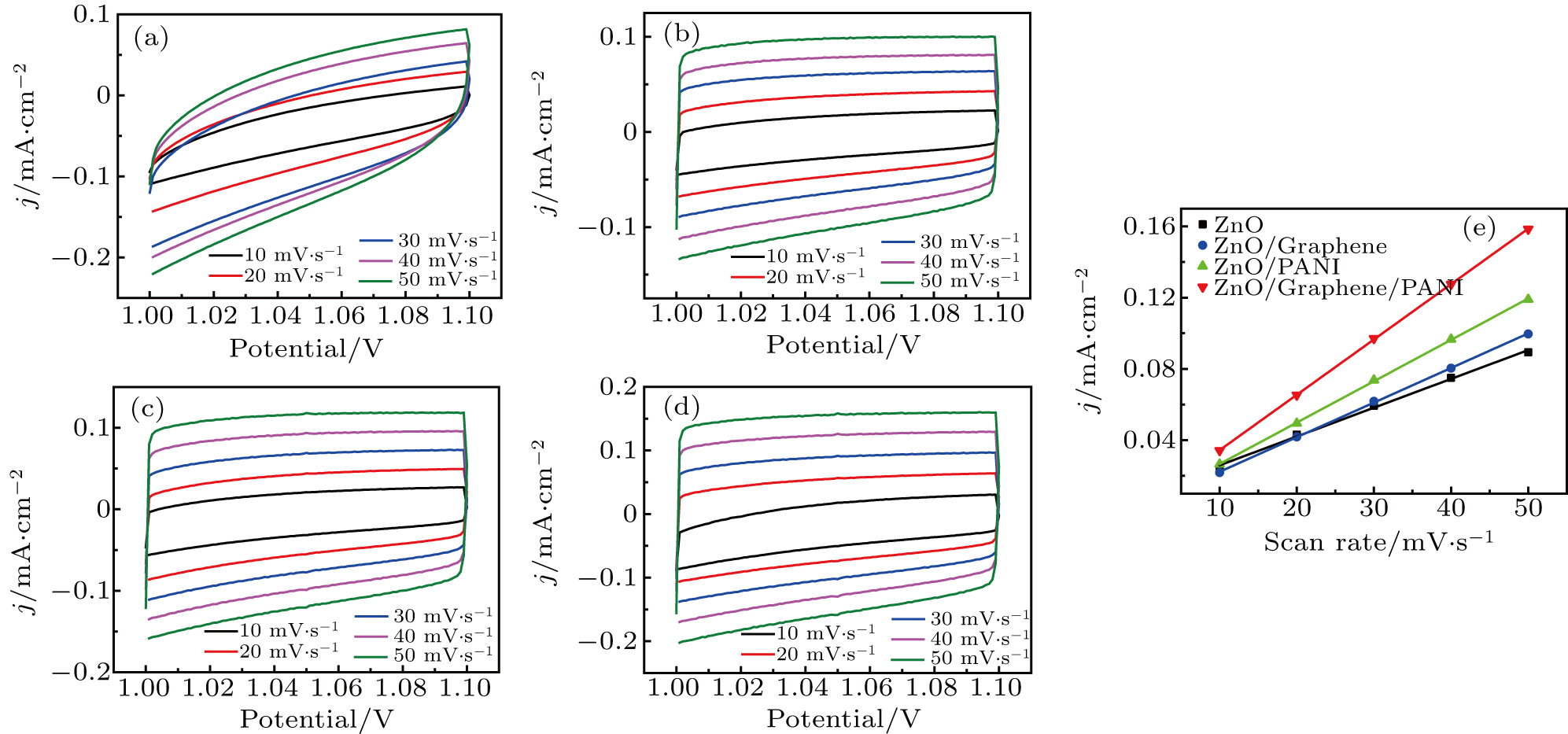 | Fig. 6. Electrochemical double layer capacitance curves on (a) ZnO, (b) ZnO/graphene, (c) ZnO/PANI, (d) ZnO/graphene/PANI with scan rates ranging from 
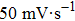
|
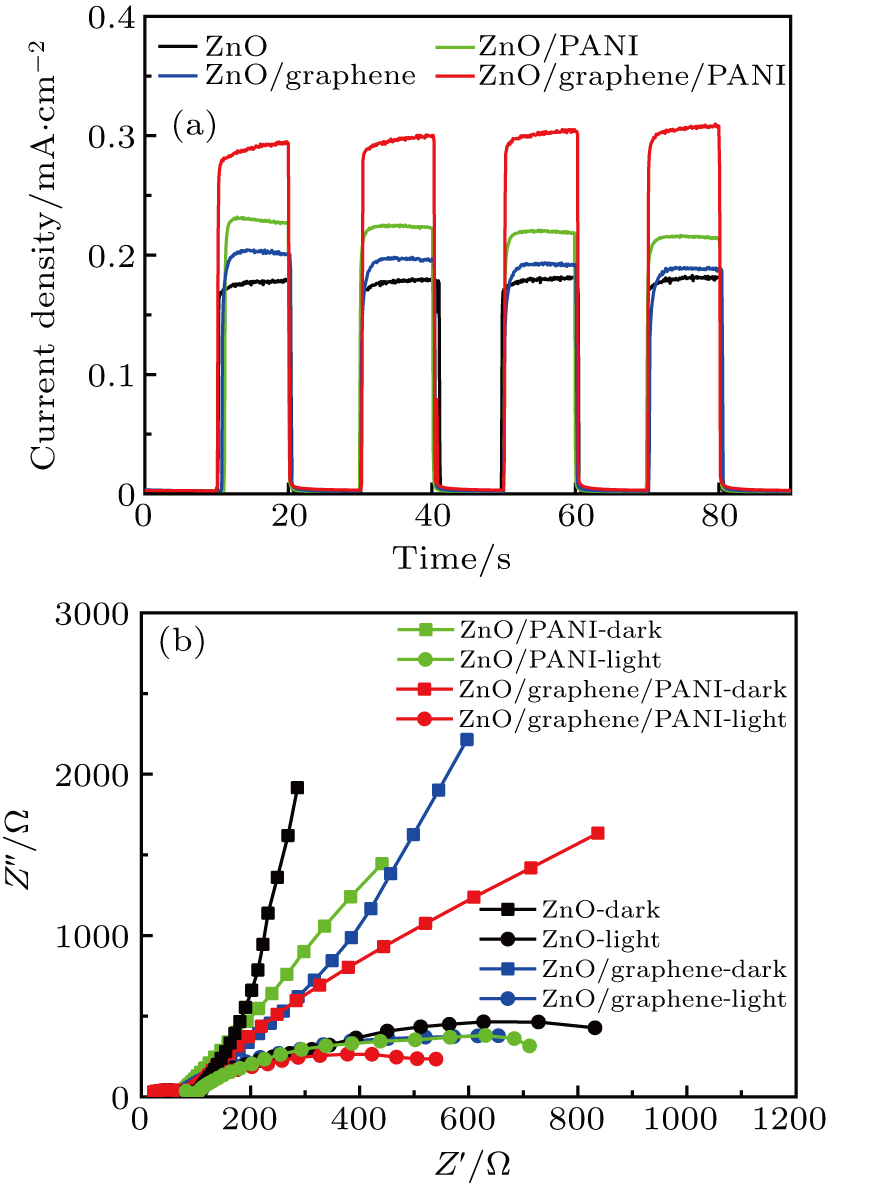
Figure 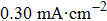



Figure
Based on the data that we have obtained, the mechanism of enhancing the photoelectrocatalytic performance for the as-prepared ZnO/graphene/PANI composite is proposed; as shown in Fig.
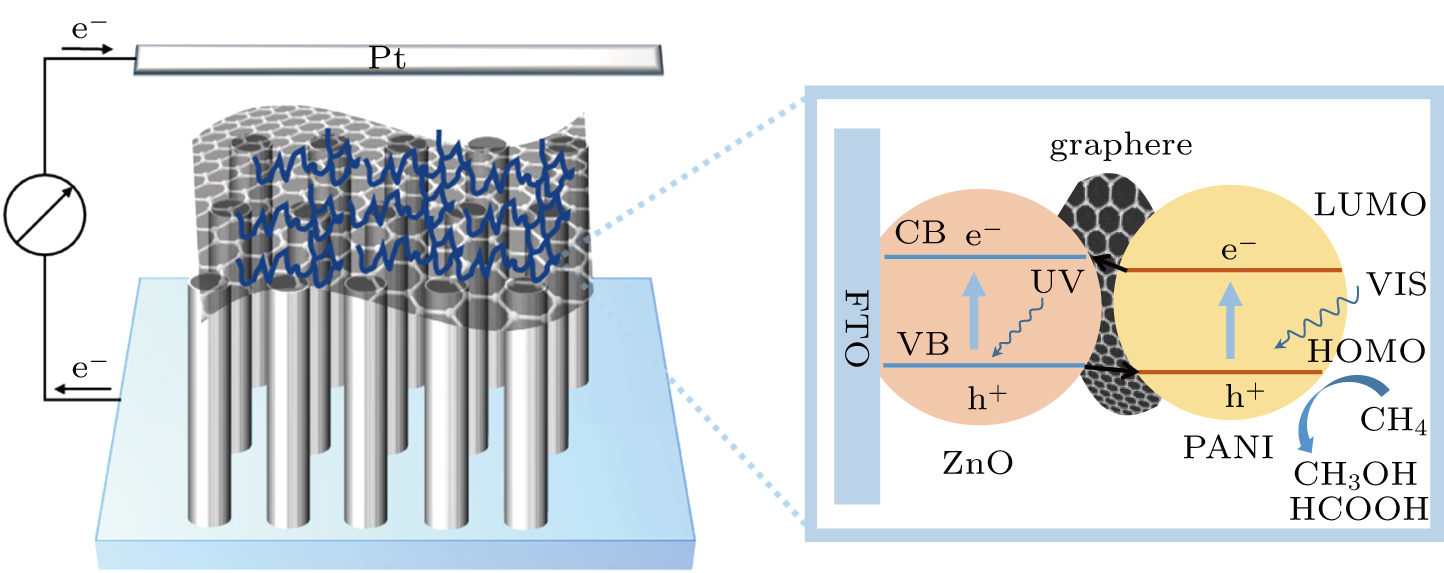 | Fig. 9. Mechanism of ZnO/graphene/PANI composite to enhance photocatalytic activity under simulated sunlight. |
The reaction shown below illustrates the electron transfer process of methane oxidation. As depicted above, with simulated sunlight illumination, ZnO/graphene/PANI composite will generate e−/h+ pairs. The positive hole 

In the present research, we report the fabrication of ZnO/graphene/PANI composite and its application as photoanode in methane oxidation. The CV curves reveal the behavior and intensity of the oxidation and reduction reactions in the entire process of methane oxidation. Moreover, the J–t and EIS curves indicate that the ZnO/graphene/PANI photoanode exhibits an enhanced photoelectrocatalytic activity under simulated sunlight illumination. The improved performance is attributed to the following crucial factors: (i) as an inter-medium layer, graphene has an exceptional ability to transfer photo-generated electrons, and thus, the photo-generated electrons in the synthesized photocatalyst can easily migrate from the inner region to the surface to participate in the surface reaction to form free radicals; (ii) the introduction of PANI extends the visible light absorption with enhanced solar energy harvesting, and promotes the electron transfer rate and the charge separation efficiency; and (iii) the PANI can create a favorable π-conjunction structure together with graphene layers, which can achieve a more effective charge separation. The research reported here shows the great potential for ZnO/graphene/PANI to serve as a photoelectrocatalyst in methane oxidation.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] |