† Corresponding author. E-mail:
The phase evolution of Bi-2223 precursor powder prepared by spray pyrolysis method is studied with different heat treatment parameters. The results show that the reaction temperature and phase composition of precursor powder depend on heat treatment atmosphere. Phase assemblage of (Bi,Pb)-2212, AEC, CuO, and small Bi-2201 can be obtained by heat-treated in N2-0.1%O2 atmosphere. For precursor powder, there is sufficient reaction process at 770 °C, and the dimension of Bi-2212 phase increases rapidly with the increase of heat treatment temperature and time. The dimension of AEC phase also increases by extending heat treatment time. As a balance among phase assemblage, dimension of particle and adequate reaction, a reasonable precursor powder can be obtained by heat-treated at 770 °C for 12 h–16 h in N2-0.1%O2 atmosphere. Critical current of 37-filament Bi-2223 tape is about 120 A, which confirms that these heat treatment parameters are reasonable.
Since the discovery of superconductivity at 30 K in the (La,Ba)2CuO4 by Bednorz and Muller,[1] many efforts have been devoted to the development of new high temperature superconductors and their applications in many different fields. (Bi,Pb)2Sr2Ca2Cu3Ox (Bi-2223) and YBa2Cu3O7 (YBCO) have become the most potential high temperature superconductors for practical application up to present, and Bi-2223 has been successfully developed into product by several companies, such as SEI, AMSC, EAS, etc.[2–4] Bi-2223 tape is fabricated by powder-in-tube (PIT) technique. Precursor powder, mechanical deformation and heat treatment are believed to be three key factors contributed to final superconducting of Bi-2223 tape. Several different preparation methods have been reported to acquire precursor powder, including solid-state reaction, oxalate co-precipitation, spray drying and spray pyrolysis.[5–7] From the viewpoint of commercialization, a reasonable preparation method should have characteristics of simple operation, good stability, high homogeneity, large quantity and good reproducibility, and spray pyrolysis is considered to be an optimal method for the industrial preparation of Bi-2223 precursor powder.
There are many studies on precursor powder in early reports. Dorris et al. and Li et al. studied the effect of two-powder process on the phase assemblage and superconducting via co-precipitation method.[8, 9] Mark et al. studied the effect of phase composition of precursor powder on Bi-2223 formation via spray drying method.[10] Jiang et al. reported the calcinations of precursor powder via solid state reaction method.[11] However, there are few studies of the phase evolution and microstructure evolution of Bi-2223 precursor powder prepared by the spray pyrolysis method.
In this work, Bi-2223 precursor powder is prepared by the spray pyrolysis method, and effects of heat treatment on phase evolution and microstructure of precursor powder are studied systematically.
We prepared precursor powder, corresponding to a nominal cation composition of Bi1.8Pb0.34Sr1.9Ca2.1Cu3.06, by the spray pyrolysis method. First, the powders were heat-treated in air and N2-0.1%O2 atmosphere separately to confirm phase forming temperature and study phase composition. Second, the powders were heat-treated at 730 °C/6 h, 750 °C/6, 770 °C/6 h, and 790 °C/6 h in N2-0.1%O2 atmosphere to study the effect of heat treatment temperature on phase composition and microstructure. Third, the powders were heat-treated at 770 °C for different times to study the effect of heat treatment time on phase composition and microstructure. Pellets of precursor powders were pressed at 20 MPa to anatomize the evolution of non-superconducting phases. Finally, 37-filament Bi-2223 tape is fabricated to confirm the reasonableness of heat treatment parameters.
The phase compositions were determined by x-ray diffraction (XRD). The morphology of powders was analyzed by scanning electron microscope (SEM). Magnetization measurements were performed using a physical property measurement system (PPMS, Quantum Design), and critical current (Ic) of tape was measured by the standard four-lead technique in nitrogen liquid with a 1-
Figure
Besides the reaction temperature, the heat treatment atmosphere affects the phase composition as shown in Fig.
Based on the results shown in Fig.
Table
| Table 1.
Lattice parameters and FWHM values of (0012) reflection of (Bi,Pb)-2212 phase in precursor powder heat-treated at different temperatures in N2-0.1%O2 atmosphere. . |
Figure 

 | Fig. 3. SEM micrographs of precursor powders heat-treated in N2-0.1%O2 atmosphere at different temperatures: (a) 730 °C/6 h, (b) 750 °C/6 h, (c) 770 °C/6 h, and (d) 790 °C/6 h. |
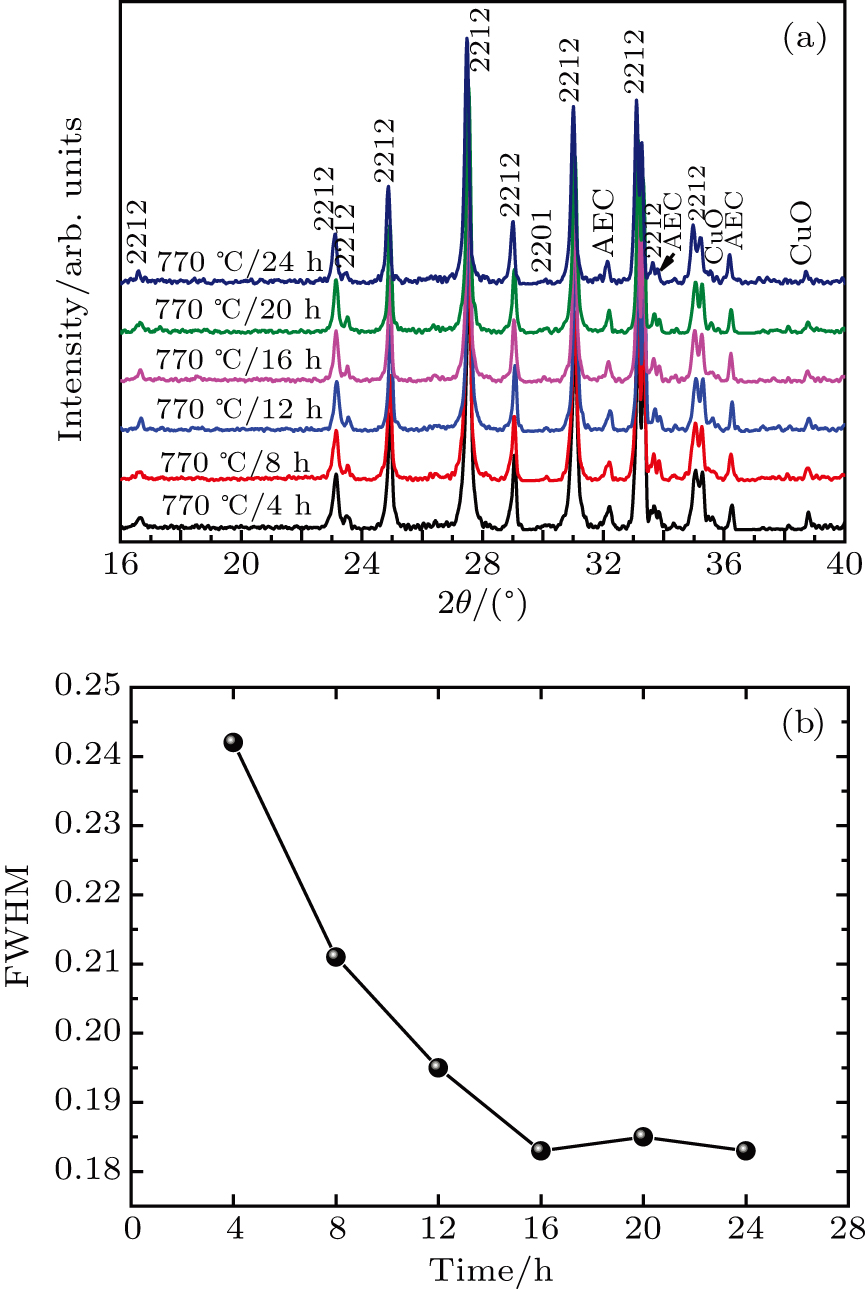
To further illuminate the effect of heat treatment time on precursor powder, the precursor powders are heat-treated at 770 °C for 4 h, 8 h, 12 h, 16 h, 20 h, and 24 h respectively. Figure
As shown in Fig. 
 | Fig. 5. SEM micrographs of precursor powders heat-treated at 770 °C in N2-0.1%O2 atmosphere for different times: (a) 4 h, (b) 8 h, (c) 12 h, (d) 16 h, (e) 20 h, (f) 24 h. |
 | Fig. 6. SEM micrographs of pellets pressed by precursor powders heat-treated at 770 °C for (a) 4 h, (b) 12 h, and (c) 24 h in N2-0.1%O2 atmosphere. |
Figure
 | Fig. 7. Plots of zero field cooled DC magnetization versus heat treatment time by PPMS in 0.5-mT field. |
Considering these results, we believe that a reasonable heat treatment process should be a balance between superconducting phase and non-superconducting phase. So it should be reasonable that precursor powder is heat-treated at 770 °C for 12 h–16 h in N2-0.1%O2 atmosphere. To confirm this viewpoint, the precursor powder is heat-treated at 770 °C/12 h in N2-0.1%O2 atmosphere and 37-filament tape is fabricated by the PIT method. The tape is heat-treated as reported elsewhere.[14] figure
We have investigated the phase evolution of Bi-2223 precursor powder with different heat treatment parameters. The main conclusions are as follows:
(i) The heat treatment atmosphere affects reaction temperature and phase composition of precursor powder. Phase assemblage of (Bi,Pb)-2212, AEC, CuO, and small Bi-2201 can be obtained by heat treatment in N2-0.1%O2 atmosphere.
(ii) With the increase of temperature, the reaction process tends to be more sufficient and the dimension of (Bi,Pb)-2212 particle size increases rapidly. The reasonable reaction temperature is believed to be 770 °C.
(iii) With the increase of heat treatment time, the difference in phase composition becomes not obvious but the dimension of (Bi,Pb)-2212 grows up obviously when heat treatment increases from 4 h to 12 h. This trend becomes less obvious for heat treatment time longer than 12 h. However, AEC phases increases rapidly with the increase of heat treatment time. It seems that the reaction process is sufficient when heat treatment time reaches to 12 h.
(iv) It should be reasonable that the precursor powder is heat-treated at 770 °C for 12 h–16 h in N2-0.1%O2 atmosphere. The critical current of about 120 A also confirms this viewpoint.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] |