Project supported by the National Natural Science Foundation of China (Grant No. 51876049) and the Fund from the Higher Education Commission, Pakistan under SRGP (Grant No. 21-1778/SRGP/R&D/HEC/2017).
Project supported by the National Natural Science Foundation of China (Grant No. 51876049) and the Fund from the Higher Education Commission, Pakistan under SRGP (Grant No. 21-1778/SRGP/R&D/HEC/2017).
† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51876049) and the Fund from the Higher Education Commission, Pakistan under SRGP (Grant No. 21-1778/SRGP/R&D/HEC/2017).
The band structure, magnetism, charge distribution, and optics parameters of TMO3–h-BN hybrid systems are investigated by adopting first-principles study (FPS) calculations. It is observed that the TMO3 clusters add finite magnetic moments to bilayer h-BN (BL/h-BN), thereby making it a magnetic two-dimensional (2D) material. Spin-polarized band structures for various TMO3–BL/h-BN hybrid models are calculated. After the incorporation of TMO3, BL/h-BN is converted into semimetal or conducting material in spin up/down bands, depending on the type of impurity cluster present in BL/h-BN lattice. Optics parameters are also investigated for the TMO3–BL/h-BN complex systems. The incorporation of TMO3 clusters modifies the absorption and extinction coefficient in visible range, while static reflectivity and refraction parameter increase. It can be surmised that the TMO3 substitution in BL/h-BN is a suitable technique to modify its physical parameters thus making it functional for nano/opto-electronic applications, and an experimental approach can be adapted to reinforce the outcomes of this study.
Since the advent of two-dimensional (2D) monolayer graphene in 2004,[1] a new series of monolayer 2D materials such as, hexagonal boron nitride (h-BN),[2] MoS2,[3] SiC,[4] and ZnO[5] have been synthesized and experimental characterizations have been performed on their monatomic states in a detailed manner. Apart from single-layered 2D material studies, a significant amount of work related to bilayer and few-layer graphene[6] has been published and the same scenario can be expected for h-BN material[7–9] in order to make it practical for novel engineering devices. Hence, comprehension of interlayer interaction in few-layered h-BN systems can produce appealing developments. Significant efforts have been made to investigate structural and energetic characteristics of mono- and multi-layered h-BN systems.[10–15] The concepts in interlayer studies include, Moiré patterns upon relative rotation of few layers of graphene[16,17] and observations of displacement in stacked layers of graphene.[18–20] These studies suggest that the physical parameters of layered 2D systems can be modified through variations in stacking position. However, another way to modify these parameters is to add impurities either in each layer or through the intercalation of impurities between layered systems. Some experimental studies were carried out on various impurity clusters intercalated few-layered graphene systems.[21–23] These studies predict that the external dopants can significantly modify the electronic, magnetic, and optics parameters of 2D materials. A recent study based on FPS–DFT (first principles study–density functional theory) method was carried out by Kaneko and Saito[24] and showed that the intercalation of alkali and alkaline earth metal atoms in bilayer graphene can modify its energy and the electrical/electronic conductivity. Likewise, FPS–DFT calculations were performed on transition metal atom intercalated bilayer graphene by Han et al.,[25] they proposed that transition metal impurities intercalation in bilayer graphene converts a nonmagnetic layered material into a stable 2D magnetic substrate. Very recently, physical properties of twisted/defective h-BN layers have been investigated,[26–29] showing that the homo/hetero/defective layers can produce functional systems for nanoelectronic and energy storage applications. It is abundantly clear that the intercalation of atoms and clusters in 2D layered systems can produce functional systems, but it requires a great deal of effort and time to design such complex materials as the interlayer distance is in nano-meter (nm) range and it is nearly impossible to induce hetero-atoms and clusters between these layers.[21,30,31] However, the adsorption or doping of impurities in few-layered systems is a viable technique to be adopted experimentally for complex layered systems.[21,32]
Like single layer h-BN, the BL/h-BN is also a wide band insulator, has a nonmagnetic nature and absorbs 2% of light in visible range.[7] In order to make BL/h-BN system functional in energy storage, optoelectronic, and spintronic applications, it is imperative to tailor the aforementioned properties. For that, we incorporate TMO3 clusters into the upper and lower layer of BL/h-BN system and investigate their effect on the electronic, magnetism, and optics characteristics of bilayer h-BN. Impurity TMO3 clusters are selected as dopants due to their charge excess and charge transport nature. These clusters can lend their charge carriers to the BL/h-BN layer, thereby modifying the energy gap in its band structure. The variation in band structure results in variations in inter and intraband transition, thus affecting the optics parameters of BL/h-BN system. Additionally, unfilled d orbitals of TM atoms produce the magnetism in the BL/h-BN, and thus making it applicable for spintronic devices. Previously, it has also been established that the halogen and super halogen, i.e., TMO3 and TMO4 clusters can greatly modify the physical parameters of monolayer graphene[33,34] and h-BN[35] systems. Hence, we try to extend this concept to few-layered h-BN systems. It can be predicted that the incorporation of TMO3 clusters into the BL/h-BN can be a viable technique to tailor its electronic, optical, magnetic, and energetic characteristics. As per our understanding, the physical characteristics of such complex systems have been scarcely studied and work on these systems remains incomplete and scattered. Outcomes of this study enable us to tune intrinsic behaviors of few-layered h-BN systems to being applicable for various technologies, in particular, opto-electronics and spintronics which are different from those of intrinsic single layer h-BN systems.
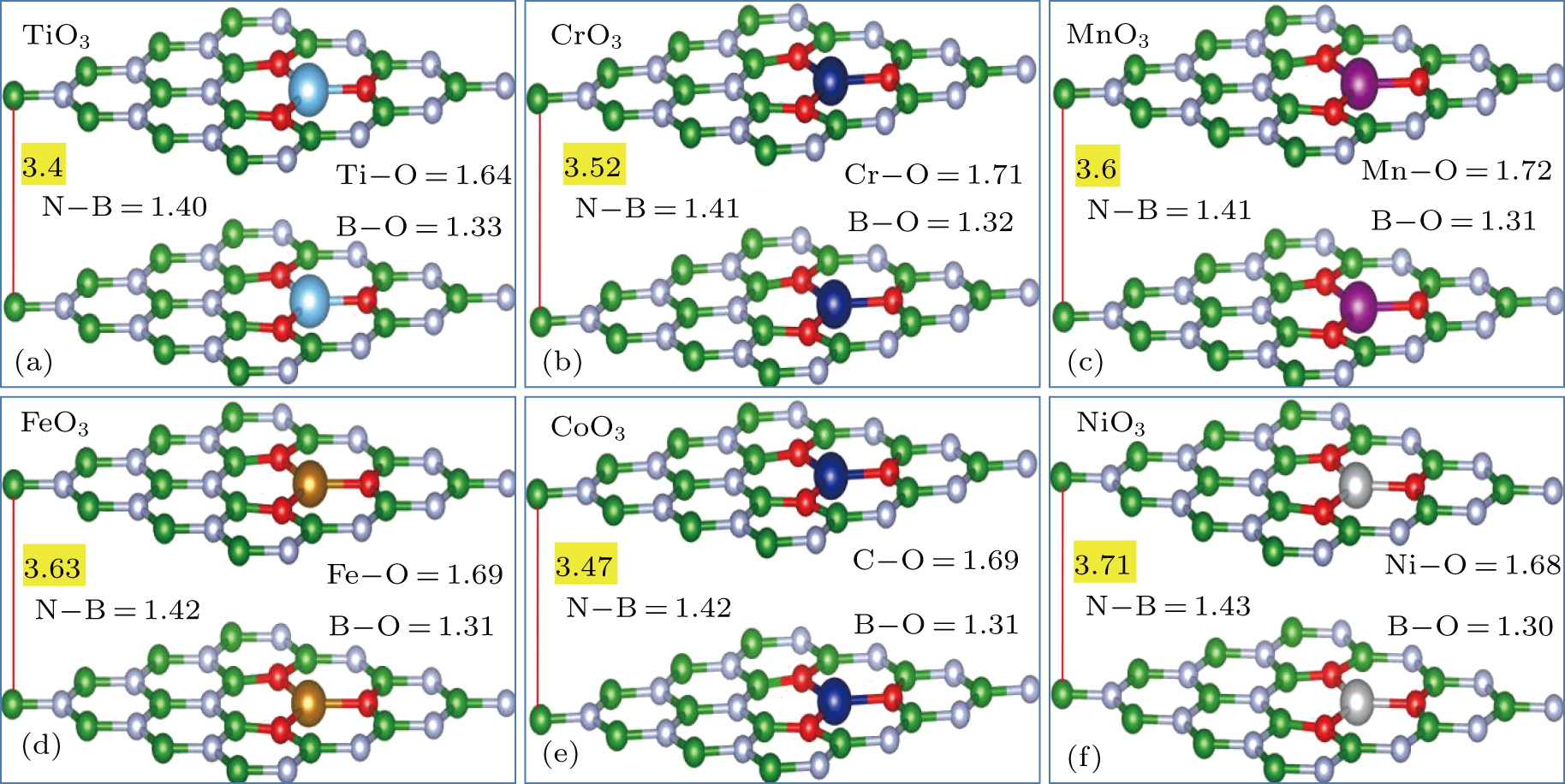
Utilizing first-principles density functional theory (FPS–DFT) within the generalized gradient approximation (GGA)[36–38] and using Vienna Ab-initio Simulation Package (VASP version 5.2.2),[39–41] the electronic structures, magnetic and optics parameters of TMO3 clusters-doped BL/h-BN were investigated. Bilayer h-BN consisted of 4 × 3 supercell structure[42,43] and the calculations were performed with plane-wave basis set including ultrasoft psuedopotentials[39,44] with cut-off energy of 450 eV. Spin polarized calculations were performed for all the parameters. For van der Waals (vdW) corrections due to the long-range vdW interactions in layered systems,[45–47] Grimme (DFT-D2)[36] method was employed. In order to remove the spurious interaction between adjacent layers, a 12-Å-thick vacuum layer was inserted along the Z direction of supercell. Various TMO3 (i.e., TiO3, CrO3, MnO3, FeO3, CoO3, and NiO3) clusters were added into upper and lower layer of h-BN and consequent effects on electronic, magnetic, and optical characteristics were investigated in a detail. To achieve good convergence criterion for various DFT results, a thick 17 × 17 × 1 k-point mesh is employed. Geometry optimization process was employed for all the complex structures until Hellmann–Feynman forces value reached 0.03 eV/Å value and total energy change less than 10−6 eV was observed. To satisfy partial occupancy criterion, Gaussian smearing technique is utilized. The top and side view of the typically relaxed structure model of pristine 4 × 3 BL/h-BN system is illustrated in Figs.
In this work, various TMO3 clusters (i.e., TiO3, CrO3, MnO3, FeO3, CoO3, and NiO3) incorporated bilayer h-BN (BL/h-BN) systems are investigated in detail by using first-principle DFT method. Physical behaviors (i.e., structure, electronic, magnetic, and optical) of pure BL/h-BN and TMO3 clusters-doped BL/h-BN systems are analyzed in detail. In order to determine the variation in atomic structure of BL/h-BN after the incorporation of TMO3 clusters, given geometries are fully optimized and all atoms are allowed to relax in the optimization process. No constraints are imposed on the constituent N/B atoms of BN sheet in the relaxation process, so that all the atoms present in BL/h-BN can reach their stable energy states.
Figures
After obtaining the structures for all TMO3 clusters-doped BL/h-BN, we investigate the formationenergy of all TMO3–BL/h-BN systems from the following expression,
 |
The formation energy values for various BL/h-BN systems, calculated from Eq. (
| Table 1.
Calculated parameters of complex TMO3 clusters-doped BL/h-BN systems: bond distances (in unit Å) of N–B (dN−B), TM–O (dTM−O), B–O (dB−O) atoms, interlayer distances (dL1−dL2 in unit Å) between h-BN sheets, total magnetic moments of the supercells (μtot, in unit μB), magnetic moments of individual TM atoms and formation energy (Ef, in unit eV). . |
In order to understand the phenomenon of charge transfer between impurity TMO3 clusters and BL/h-BN layer, the differences in charge are calculated from all given models through Bader analysis.[55,56] The charge transfer of TMO3–BL/h-BN (i.e., Δ ρTMO3−BL/h−BN) complex systems can be determinedfrom the following expression:
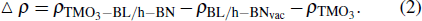 |
Utilizing expression (2), charge difference diagrams of various TMO3 (i.e., TiO3, CrO3, MnO3, FeO3, CoO3, and NiO3) clusters-doped BL/h-BN systems can be calculated and the results are presented in Figs.
As listed in Table
In this subsection, the electronic structures of all TMO3 clusters-doped BL/h-BN systems are obtained. For electronic parameter calculations, 30 points are used for M–Γ–K–M path in the irreducible Brillouin (IB) zone, in order to achieve band structure with a finer grid. A 19 × 19 × 1 k-points grid is employed to obtain the density-of-states (DOSs) plots. A Gaussian distribution of width of 0.02 eV is used for smearing eigenvalues. Total and partial DOSs of B and N atoms of BL/h-BN system, O and TM atoms of TMO3 clusters are calculated for all given systems. Owing to the reservations generated for GGA–PBE and hybrid functional predictions, GGA and LDA systems are utilized separately for a test run, in order to establish band gap sensitivity on these functionals. It is revealed that the band gap value is not heavily dependent on the choice of basis set, and a trivial difference is observed to be ∼ 0.03 eV in the band gap value.
Before extracting the electronic structures for various TMO3 clusters-doped BL/h-BN systems, the electronic structure of pure BL/h-BN system is calculated and compared with earlier study as shown in Figs.
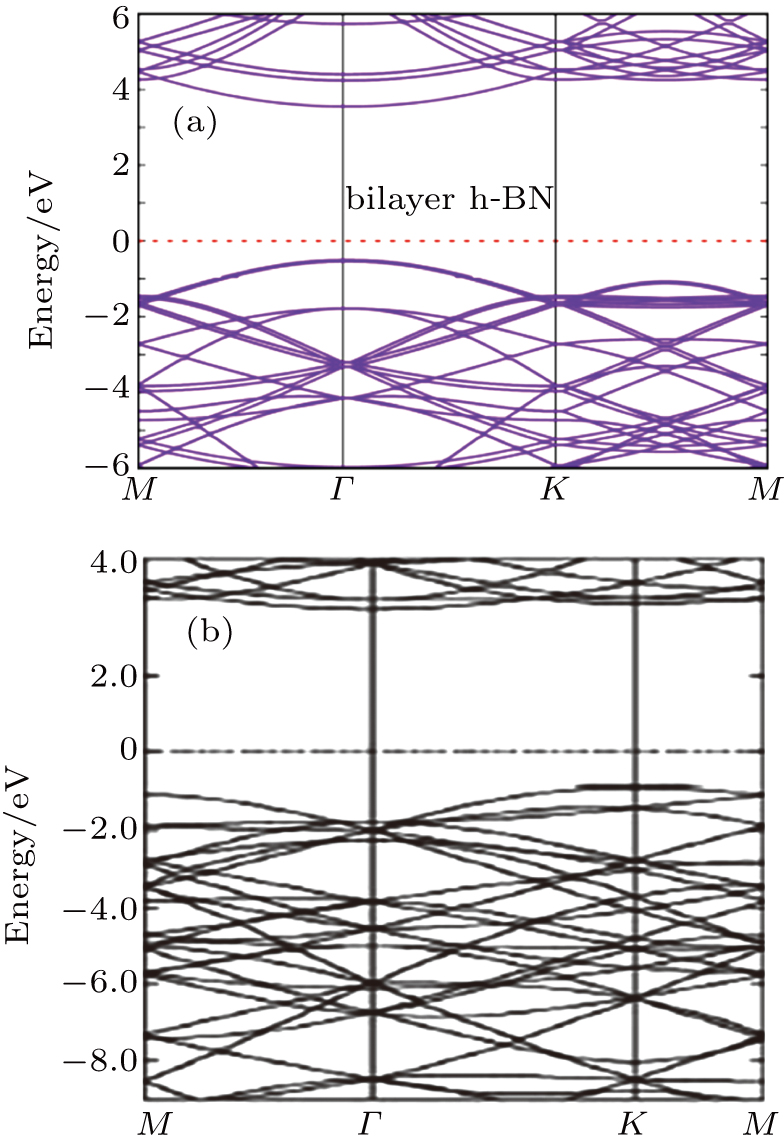 | Fig. 5. Electronic structure of pure BL/h-BN system (a) obtained from this work and (b) cited from Ref. [29]. |
Donor impurity TMO3 clusters, when incorporated into BL/h-BN, can significantly modify its electronic structure due to the difference in energy between charge carriers of TMO3 clusters and the BL/h-BN.[33,34,64]
Spin-polarized electronic structures of various TMO3 (i.e., TiO3, CrO3, MnO3, FeO3, CoO3, and NiO3) clusters-substituted BL/h-BN systems are described in Figs.
In order to determine the orbital interaction between TMO3 clusters and BL/h-BN, the spin-polarized total and partial DOS (T/P-DOS) in given TMO3 clusters-doped BL/h-BN systems are calculated and analyzed. Resulting T/P-DOS for TM atoms, neighboring B/N atoms p orbitals and O atom p orbitals for all TMO3 clusters-incorporated BL/h-BN systems are illustrated in Figs.
Now, we come to investigate the optical parameters of pure and TMO3 clusters-doped BL/h-BN systems. The absorption coefficient α, extinction coefficient k, refractive index n, and reflectivity R are obtained and analyzed through DFT by the random phase approximation (RPA)[66] technique, in which local field effect is neglected. Only interband transitions are included, so there can be some inaccuracies in dielectric function at low energy. We have utilized this technique in our earlier studies and found it reliable and accurate enough to extract the optical parameters of defective 2D layered systems.[42,51,64,67] These optical parameters are extracted from the dielectric constant quantity, which is the sum of real and imaginary parts, i.e., ε = ε′ + ε″. Firstly, the imaginary part (ε″) is gained through the summation of empty states as follows:[42,68]
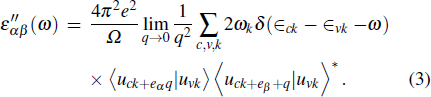 |
Secondly, using Kramers–Kronig transformation, the real part of dielectric (ε′) is gained as follows:
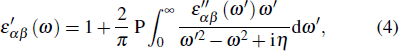 |
From the value of real and imaginary parts of dielectric tensor we can easily obtain refractive index n and extinction coefficient k by using the following equations:
 |
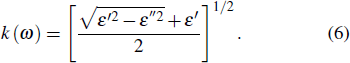 |
 |
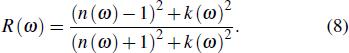 |
The values of refractive index n and extinction coefficient k parameter of the pure and TMO3 clusters-doped BL/h-BN systems are obtained and shown in Figs.
 | Fig. 8. Plots of (a) refractive index n and (b) extinction coefficient k parameter versus energy of pristine and TMO3 clusters-doped BL/h-BN systems. |
A significant variation is gained in the extinction coefficient k of pristine BL/h-BN after incorporating the TMO3 clusters, i.e., a shift in the first extinction peak towards the lower energy range, thus producing a red shift. As seen in Fig.
The plots of absorption coefficient α and reflectivity R versus energy for pure and TMO3 clusters-doped BL/h-BN systems are shown in Figs.
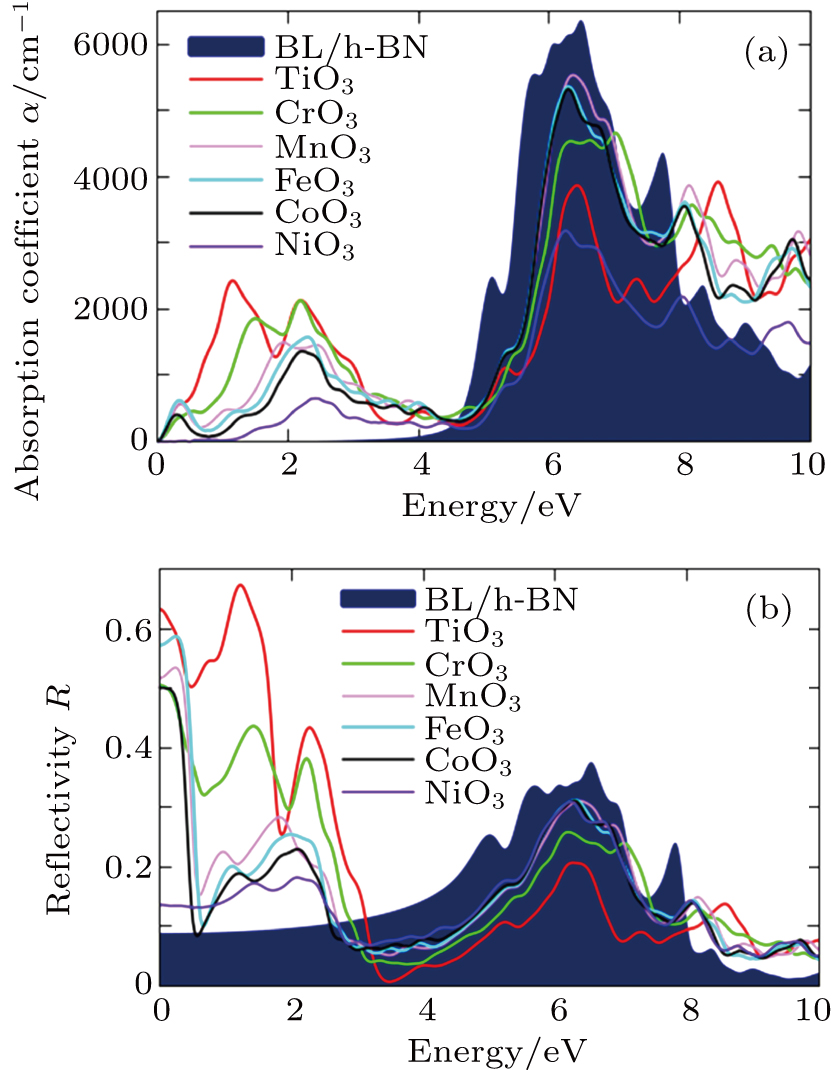 | Fig. 9. Plots of (a) absorption coefficient α and (b) reflectivity R parameter versus energy of pristine and TMO3 clusters-doped BL/h-BN systems. |
From the absorption coefficient α plot of Fig.
Finally, the reflectivity R parameter for each of pure and TMO3 clusters-doped BL/h-BN system is analyzed and shown in Fig.
Adopting first-principles density functional theory, opto-electronic and magnetic behaviors of TMO3 cluster-incorporated bilayer h-BN (BL/h-BN) systems are studied and analyzed in detail. Different TMO3 clusters (i.e., TiO3, CrO3, MnO3, FeO3, CoO3, and NiO3) clusters are embedded respectively in upper and lower layers of BL/h-BN and their corresponding effects on aforementioned physical properties are investigated. Formation energy, charge transfer, and spin density parameters are also calculated for all the systems. Positive formation energy is obtained for each of all TMO3 clusters-doped BL/h-BN systems, indicating an exothermic stable thermodynamic process. The charge transfer analysis reveals that the TMO3 clusters are donor impurities, thereby suggesting that they possess the n-type doping behavior. The TiO3, MnO3, FeO3, and CoO3 clusters-doped BL/h-BN systems possess the magnetic moments of 3.6 μB, 6.0 μB, 4.0 μB, and 2.0 μB, respectively, hence larger electron spin variation is observed at the defect site. These TMO3 clusters greatly modify the band structure of BL/h-BN system. The spin-polarized band structures obtained for all TMO3 clusters-doped BL/h-BN systems indicate that the BL/h-BN system displays half metal/conductor/semiconductor behavior in either spin-up or spin-down band, thereby making these systems useful for the spintronic devices. It is interesting to note here that the band gaps in electronic structures appear at different energy levels subjected to the polarization of energy bands, and the band gap opening is sensitive to the type of impurity cluster present in the BL/h-BN lattice. From the spin-polarized total and partial DOS plots, it can be predicted that the unfilled d orbitals of TM atoms are due mainly to the magnetism in BL/h-BN and the stronger magnetic coupling generated between TMO3 clusters and BL/h-BN system. The optical parameter calculations reveal that the values of static refractive index and reflectivity parameters of BL/h-BN increase after TMO3 clusters have been added into its lattice, while minor variations are observed in the above parameters in a higher energy range. Likewise, the extinction and absorption coefficient parameters of BL/h-BN layer are greatly improved in a lower energy range and new peaks appear after TMO3 clusters have entered into its lattice. It is important to note that none of new emerging peaks is available in the optical spectrum of pure BL/h-BN layer, suggesting a red shift towards visible range of spectrum in extinction and absorption coefficients, thus making these TMO3–BL/h-BN systems applicable for solar energy devices. Outcomes of this study provide a suitable and thermodynamically stable technique to tailor the opto-electronic and magnetic behaviors of BL/h-BN systems, also suggest that the synthesis of few layer h-BN materials is functional for spintronic and smart energy applications.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] | |
| [62] | |
| [63] | |
| [64] | |
| [65] | |
| [66] | |
| [67] | |
| [68] | |
| [69] | |
| [70] | |
| [71] | |
| [72] | |
| [73] | |
| [74] | |
| [75] | |
| [76] |