Project supported by the Natural Science Foundation of Anhui Province, China (Grant Nos. KJ2018A0588 and KJ2019A0879).
Project supported by the Natural Science Foundation of Anhui Province, China (Grant Nos. KJ2018A0588 and KJ2019A0879).
† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Anhui Province, China (Grant Nos. KJ2018A0588 and KJ2019A0879).
Density functional theory calculations are carried out to identify various configurations of oxygen molecules adsorbed on the Au-doped RuO2 (110) surface. The binding energy calculations indicate that O2 molecules are chemically adsorbed on the coordinatively unsaturated Ru (Rucus) sites and the bridge oxygen vacancies on the Au sites. Transition state calculations show that O* can exist on the Rucus site by 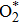
The physical and chemical properties of transition metal oxides are of great interest and importance for efficiently using catalysis, electrochemistry, and gas sensors. Because the surfaces of these oxides perform well compared with the bulk state, the important goal in such studies is to reveal the surface functionality, especially their interaction with a gas phase species on anatomic scale.[1,2]
In recent years, ruthenium dioxide (RuO2) has become a popular and convenient model system for investigating catalytic reactions. And CO oxidation has attracted much attention because it plays an important role in solving the exhaust gas issues generated from auto-mobiles and industries. In CO oxidation, the surface of metallic Ru is inactive, but after being highly exposed to O2, it displays excellent performance due to the formation of a RuO2 (110) film.[3–6] A Mars–van Krevelen mechanism and a Langmuir–Hinshelwood model were successively proposed for the explaining of the CO catalytic oxidation on the chemical surface.[7,8]In the Mars–van Krevelen model, reductants react with metal oxide lattice oxygen, and then the dissociative adsorption of O2 fills the resulting oxygen vacancies. The Langmuir–Hinshelwood model suggests that CO combines with dissociated O* into CO2. Therefore, O2 adsorption and dissociation are important processes for catalytic reactivity.[9,10] Recent studies have experimentally and theoretically reported that oxygen molecules are adsorbed on the stoichiometric RuO2 (110) surface.[11,12] On the other hand, to improve the properties of a catalyst, an effective strategy is to modify the metal oxides with other metal elements. For example, the (111) surface of ceria can be made reactive to CO oxidation by doping the surface with Au theoretically.[13] Because doped gold atoms become gold ions which play an important role in determining the CO catalytic oxidation, gold becomes a significant doping element. Therefore, it is interesting to study the oxygen adsorption and CO oxidation on Au-doped RuO2 (110) surface.
In this study, O2 adsorption, dissociation, and diffusion on the Au-doped Ru (110) surface are investigated by using density functional theory. First, the optimized configurations of molecular O2 adsorbed on the Au-doped RuO2 (110) surface are obtained. Then the adsorption energy and dissociation energy barrier are calculated. The reaction paths of CO adsorbed onto Au with the O* atom are also determined.
Calculations based on the density functional theory (DFT) were analyzed in the generalized gradient approximation (GGA)[14] by using the Perdew–Burke–Emzerhof function for correlation energy. Plane-wave basis functions were included with a kinetic energy cutoff of 450 eV. The calculations were carried out by using the Brillouim zone sampled with 4 × 4 × 6 and 2 × 2 × 1 Monkhorst–Pack mesh k-points grid for bulk RuO2 and surface calculations, respectively. The structures were relaxed by using the BFGS algorithm until the forces on all atoms were <0.02 eV·Å−1. The first-principles calculations were performed by using the Cambridge Sequential Total Energy Package (CASTEP) code.[15]
Figure
Oxygen dissociation and diffusion barriers were calculated by using the Climbing Image Nudged Elastic Band (Cl-NEB) method.[17,18] Five intermediate images were generated between the geometry-optimized initial and final state by linear interpolation. Minimum energy reaction paths were obtained by simultaneously relaxing each image. Vibrational frequencies were calculated through the diagonalization of the Hessian matrix by displacing the atoms by 0.01 Å from their equilibrium positions. The adsorption energy of molecular O2, the oxygen vacancy formation energy, and the charge density difference were calculated below.
The adsorption energy was evaluated from
 |
The oxygen vacancy formation is described by the reaction 
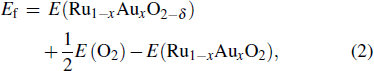 |
The charge density difference ρdiff was obtained from the following expression
 |
RuO2 crystallizes into a rutile structure with lattice parameters a and c. To ensure the reliability of the computational results, the bulk lattice constants are predicted and compared with the experimental value. The calculated lattice parameters of the pure RuO2 are a = 4.53 Å and c = 3.13 Å, which accord with the results of Sun (a = 4.52 Å and c = 3.13 Å) obtained through a DFT–GGA pseudopotential study.[20] The theoretical values are similar to the experimental values of 4.492 and 3.306.[21,22] The geometries of stoichiometric and reduced-bulk Au-doped RuO2 are optimized until the external and internal degree of freedom can relax to the force and stress vanished in the bulk calculations. The lattice constants change after the Au atoms have been doped into the bulk RuO2, the predicted lattice constants of bulk 12.5% Au-doped RuO2 are a = 4.58 Å and c = 3.20 Å, which are slightly larger than those of pure RuO2 because of larger radius of the Au atom. The optimized Ru0.875Au0.125O2 presents geometric distortion with an average length of the Ru–O bonds, 1.988 Å, while that of Au–O bonds is 2.163 Å. The RuO2 (110) surface that is built below will use these lattice constants.
The relaxed structure of the Au-doped RuO2 (110) surface in which one Ru atom is replaced by one Au atom is shown in Fig.
As is well known, O2 can bind to the transition metal center. In the present study, there are four sites for O2 adsorption: Rucus, Au, and O vacancies A and B as shown in Fig.
Figure 

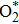
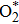


 | Fig. 2. Representative configurations and charge density differences of molecular oxygen adsorption involving a br vacancy, cus and Au sites. |
| Table 1.
Values of adsorption energy (ΔEO2), bond length (dO−O), Bader charge of O2 ( |
According to Wang et al.’s study,[16] O2 can be adsorbed molecularly across two adjacent coordinatively unsaturated vacancies. In the present study, O2 is adsorbed molecularly across Rucus and Au as shown in Fig. 



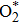
Next, we consider O2 adsorbed across adjacent vacant bridge A or B and Rucus or Au site as shown in Figs. 


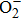
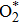
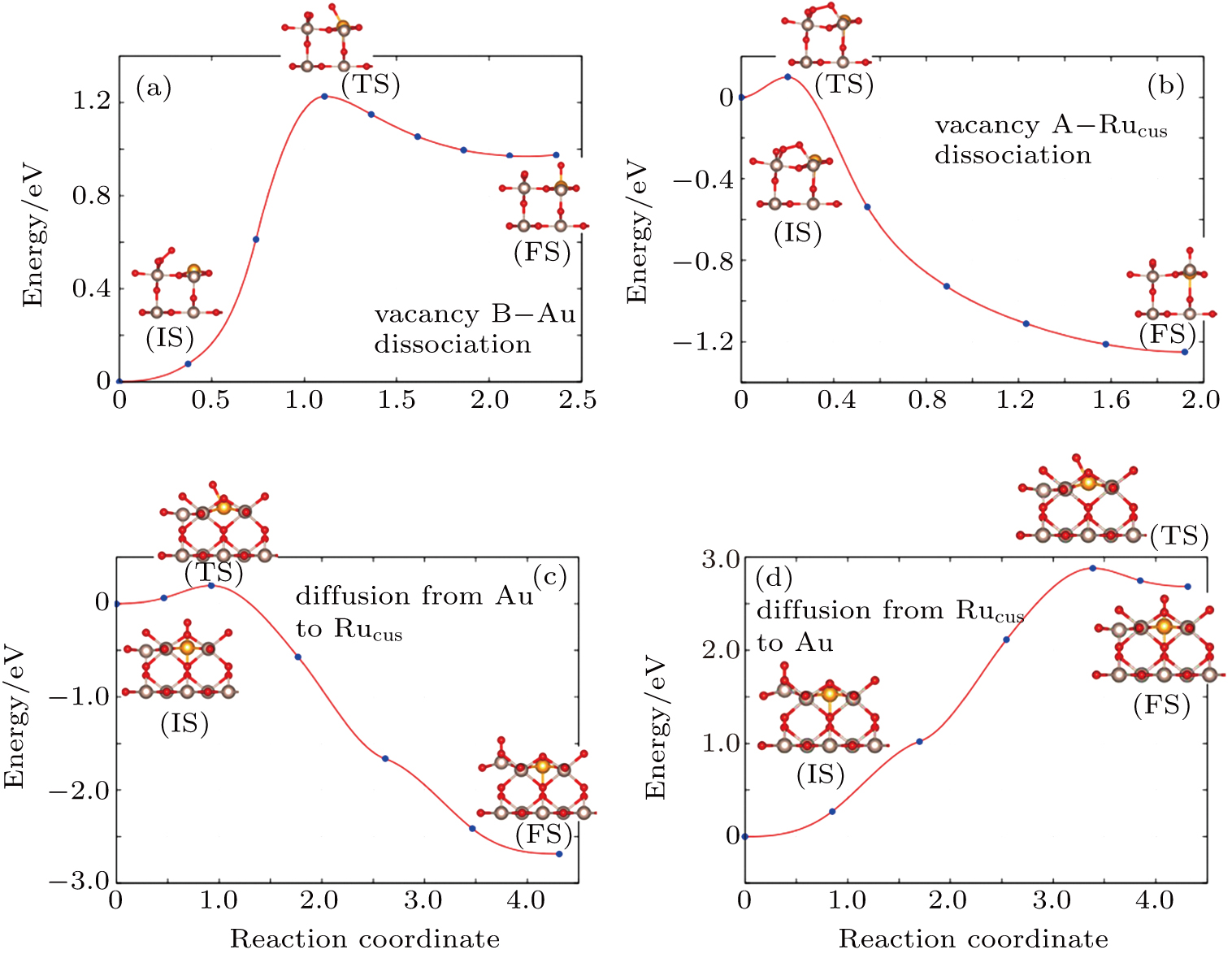
The minimum energy path from molecular 
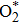

The dissociation of molecular 
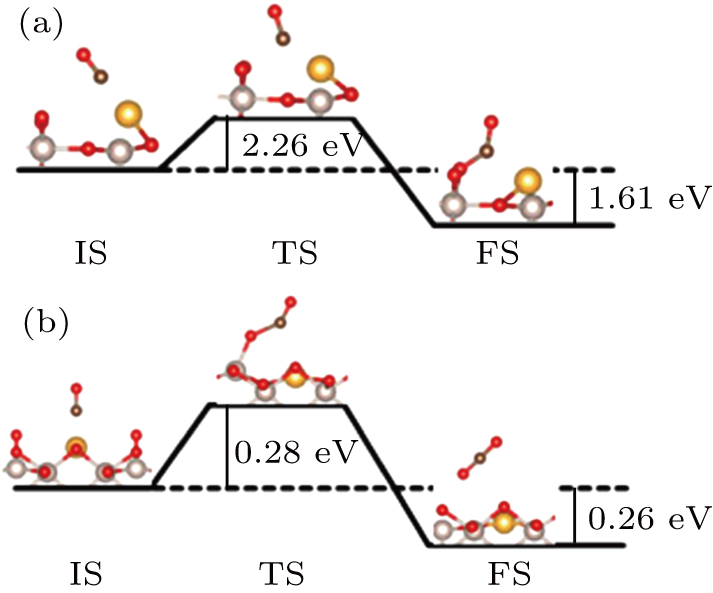
The experimental study indicates that CO oxidizes readily into CO2 on the RuO2 (110) surface,[26–28] which is also corroborated by many theoretical investigations.[29,30] Previous studies show that CO is first adsorbed on the stoichiometric RuO2 (110) surface, then reacts with the O-bridge atom. Finally, the CO2 is desorbed with an O-bridge vacancy remaining. In the present study, two CO oxidation processes happening on the Au-doped RuO2 (110) are considered. One is that the CO adsorbed on Au reacts with O-bridge to form CO2, and the other is that the CO on Au combines with the O* adsorbed on the neighboring Rucus. The calculated relevant energy values and reaction paths are summarized in Fig.
The DFT calculation is carried out to describe the molecular oxygen adsorption and reaction chemistry of CO on the Au-doped RuO2 (110) surface. The O2 can be adsorbed on Rucus, Au, and oxygen bridge vacancies with different configurations. In comparison, the O2 binding energy at the oxygen bridge vacancies is the largest, exceeding −6.98 eV, while the binding energy at the Au atom is only −0.44 eV. And it is found that the configurations can all dissociate at available neighboring vacant sites, which leaves a single O* adsorbed at the Rucus and Au site. The calculation results indicate that the dissociated O* is easier to diffuse from Au to Rucus site than from Rucus to Au. Finally, the CO reaction paths are obtained by DFT. The results show that the reaction energy barrier of CO adsorbed at Au with lattice oxygen decreases to 0.28 eV. However, the reaction of CO with O* at Rucus site requires more energy.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] |