Project supported by the National Natural Science Foundation of China (Grant Nos. 11705246, 11675233, and 11690041) and the Natural Science Foundation of Gansu Province, China (Grant No. 17JR5RA316).
Project supported by the National Natural Science Foundation of China (Grant Nos. 11705246, 11675233, and 11690041) and the Natural Science Foundation of Gansu Province, China (Grant No. 17JR5RA316).
† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11705246, 11675233, and 11690041) and the Natural Science Foundation of Gansu Province, China (Grant No. 17JR5RA316).
Polycrystalline samples of La2Zr2O7 pyrochlore are irradiated by different energetic heavy ions to investigate the dependence of the vibrational mode variations on the irradiation parameters. The applied electronic energy loss (dE/dx)e increases from about 5.2 keV/nm to 39.6 keV/nm. The ion fluence ranges from 1 × 1011 ions/cm2 to 6 × 1015 ions/cm2. Vibrational modes of irradiated pyrochlore are analyzed by using Raman spectrum. Infrared active modes F1u at 192, 308, and 651 cm−1 appear in Raman spectra, and the F2g band at 265 cm−1 rises up due to the irradiation by 200-MeV Kr ions with (dE/dx)e of 16.0 keV/nm. Differently, for the pyrochlore irradiated by 1750-MeV Bi ions with (dE/dx)e of 39.6 keV/nm, in spite of the appearance of infrared active mode F1u 651 cm−1, the amorphous structure occurs according to the vibrational mode variations of pyrochlore irradiated at higher ion fluences. Amorphous tracks are observed in the samples, which confirm the occurrence of pyrochlore–amorphous transition in pyrochlore irradiated with (dE/dx)e of 39.6 keV/nm.
Pyrochlore, as a fast-ion conductor for electrolytes in solid oxide fuel cells,[1] frustrated magnetism,[2] ferroelectricity,[3] photocatalys,[4] photoluminescence,[5] has aroused the great interest of all. Besides, A2B2O7 pyrochlore has great attraction in present nuclear technology as a material for disposal of nuclear wastes, due to its ability to accommodate minor actinides and an excellent resistance to radiation in the nuclear stopping regime. Pyrochlore has a superstructure of the fluorite structure-type with the A-site and B-site cations ordered and one-eighth of the anions missing.[6,7] Two different oxygen sites occur in pyrochlore: the 48f sites (0.75-x, 1/8, 1/8) that are occupied by six oxygen atoms (O), and the 8a site (1/8, 1/8, 1/8) where the seventh oxygen atom (O′) is located.[6–8] Fantastic properties of pyrochlore originate particularly from the presence of one vacant site per eight oxygen sites that could provide fast channels for oxygen diffusion.[8]
The response of pyrochlore to the extreme conditions has received a great deal of attention. Heavy ion irradiation is usually used to simulate harsh irradiation environment of materials.[9–11] Heavy ion irradiation has been applied to pyrochlore for precision micromachining, forming nanostructures, processing thin films, and inducing phase transitions. Interestingly, it is found that the phase transition and the microstructure during heavy ion irradiation are varied with the chemical composition of pyrochlore.[12–16] Quite a lot of researches have been conducted on the binary Gd2Zr2–xTixO7 pyrochlore.[17–20] It is indicated that the resistance to irradiation-induced amorphization significantly increases with the Zr-content. Rare earth titanate Gd2Ti2O7 undergoes a pyrochlore-to-amorphous transition. Whereas Gd2Zr2O7 is transformed into an anion-defect fluorite structure. Actually, the cationic radius ratio rA/rB of pyrochlore governs the stability of the pyrochlore structure.[16,21–23] It is reported that in the ambient conditions, the pyrochlore is stable as its rA/rB ranges from 1.46 to 1.78. A disordered fluorite structure can be formed as the rA/rB drops below 1.46. While the structure will exhibit a monoclinic, layered-perovskite structure when its rA/rB is above 1.78.[21] The cation ionic ratio rA/rB of La2Zr2O7 pyrochlore is about 1.61. Chartier et al. have shown that La2Zr2O7 has a greater tendency toward cation disorder.[24] It is thought that La2Zr2O7 should be “resistant” to radiation damage by simply disordering to the defect fluorite structure rather than becoming amorphous structure.[14,24] Moreover, physical properties of the materials are strongly dependent on the structural stability. Sattonnay et al. found that the evolution of the mechanical properties of swift heavy ion irradiated pyrochlore depends on the residual stress introduced into the sample surface layer.[25,26] Sellami et al. observed that the thermal conductivity decreases in irradiated Gd2Ti2O7 and Y2Ti2O7.[27] In this work, La2Zr2O7 pyrochlore is exposed to energetic heavy ion irradiation with different ion fluences and (dE/dx)e varying from 5.2 keV/nm to 39.6 keV/nm. The vibrational modes of irradiated La2Zr2O7 pyrochlore are investigated to find out the dependence of structural transition on irradiation parameters.
La2Zr2O7 pyrochlore pellets were prepared by a sol–gel method with an aqueous mixture of zirconium nitrate solutions Zr(NO3)4 · 3H2O and appropriate rare-earth nitrate La(NO3)3 · 6H2O. Citric acid was used as the complexing agent. Each standard solution was accurately measured and mixed according to Table
| Table 1. Detailed chemical design for La2Zr2O7 pyrochlore. . |
La2Zr2O7 samples were irradiated at room temperature with various kinds of heavy ions (e.g., Ar, Kr, Bi) accelerated by the heavy ion research facility in Lanzhou (HIRFL) at fluences ranging from 1 × 1011 ions/cm2 up to 6 × 1015 ions/cm2. The ion flux was always kept lower than 5 ions/(cm2 · s)–8 × 108 ions/(cm2 · s) to avoid overheating. The electronic energy loss (dE/dx)e near the surface of the irradiated La2Zr2O7 increased from about 5.2 keV/nm to 39.6 keV/nm. All irradiated experiments were performed in vacuum at room temperature. The detailed parameters, e.g., (dE/dx)e, were calculated by SRIM 2010 code,[28] as shown in Table
| Table 2. Detailed parameters applied in this experiment. . |
A Microscopic confocal Raman spectrometer (Lab RAM HR800, Jobin Yvon Co.) was used to analyze the vibrational modes of irradiated La2Zr2O7 pyrochlore at room temperature. The laser excitation wavelength was 532 nm. Raman shift from 150 cm−1 to 1000 cm−1 was chosen for this experiment. The total exposure time was 30 s for each sample.
Focus ion beam system (FIB, Helios Nanolab 600, FEI) was used to prepare the samples for cross-sectional observation of microstructure. The depth of the prepared sample was about 5.5 μm from the irradiated surface. The specimen prepared by FIB was examined by using transmission electron microscope (TEM, 2100 F, JEOL) to observe the profiles of the nano structure of the irradiated samples. The TEM was operated at 200 kV.
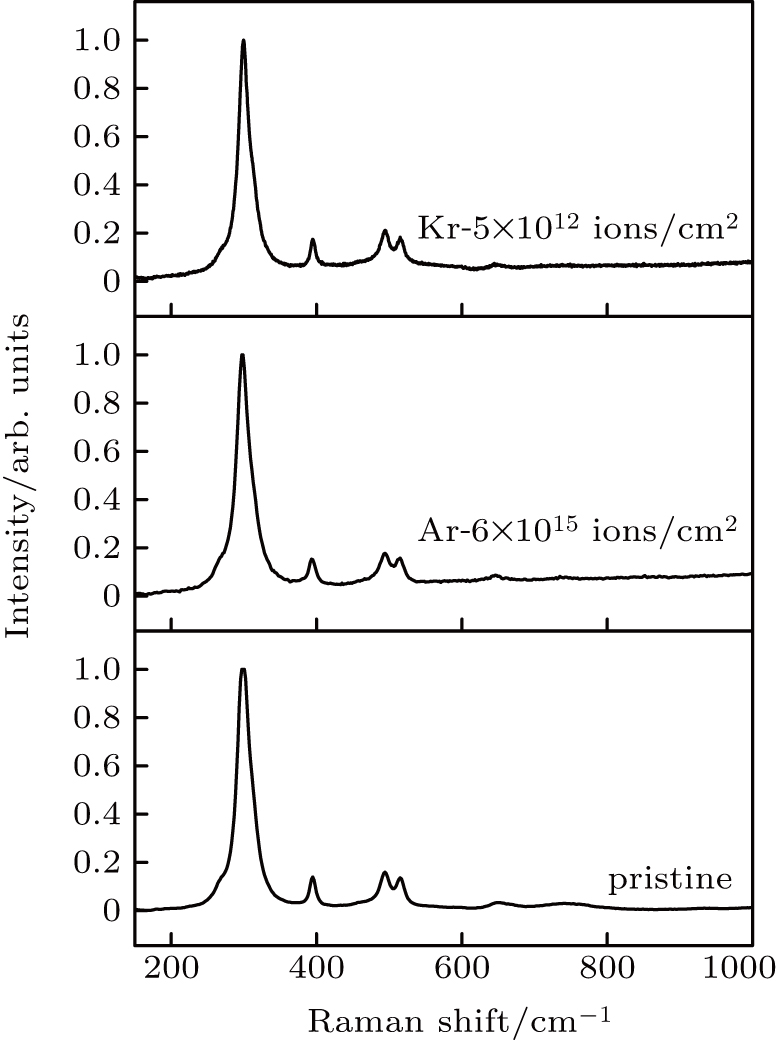
Raman spectroscopy is highly sensitive to metal-oxygen vibrational modes. In Raman spectrum, La and Zr atoms in La2Zr2O7 pyrochlore do not contribute to the Raman active vibrations because they are located at the inversion center.[6,29] Raman activity of pyrochlore results from oxygen vibrations. The Raman spectrum of La2Zr2O7 pyrochlore is similar to that of other A2B2O7 pyrochlore with six Raman active vibrational modes. They are A1g + Eg+4F2g. Five obvious bands are observed in the Raman spectra of pristine La2Zr2O7 as shown in Fig.
In this work, heavy ions with different energy are used to irradiate La2Zr2O7 pyrochlore to investigate the influences of irradiation parameters on vibrational modes’ variations of La2Zr2O7. It is observed that no substantial changes appear on the main vibrational modes when La2Zr2O7 is irradiated by 260 MeV Ar and 1980-MeV Kr ions with (dE/dx)e of 5.2 keV/nm and 9.1 keV/nm, respectively, and the results are shown in Fig.
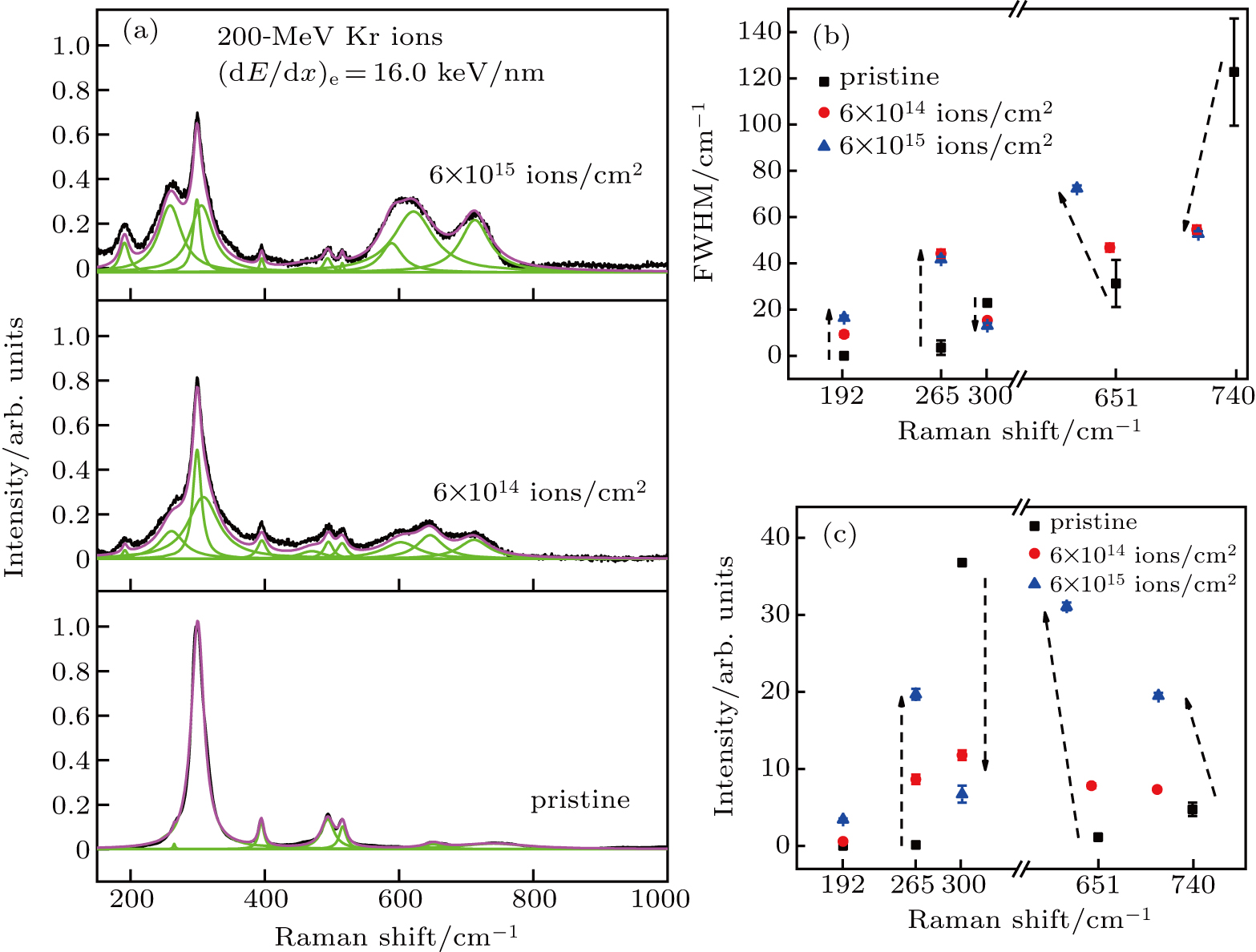
Figure
According to Fig.
The influences of irradiation parameters on the vibrational modes of La2Zr2O7 pyrochlore are further verified. The La2Zr2O7 pyrochlore sample is subjected to the irradiation of 1750-MeV Bi ions ((dE/dx)e = 39.6 keV/nm). Figure
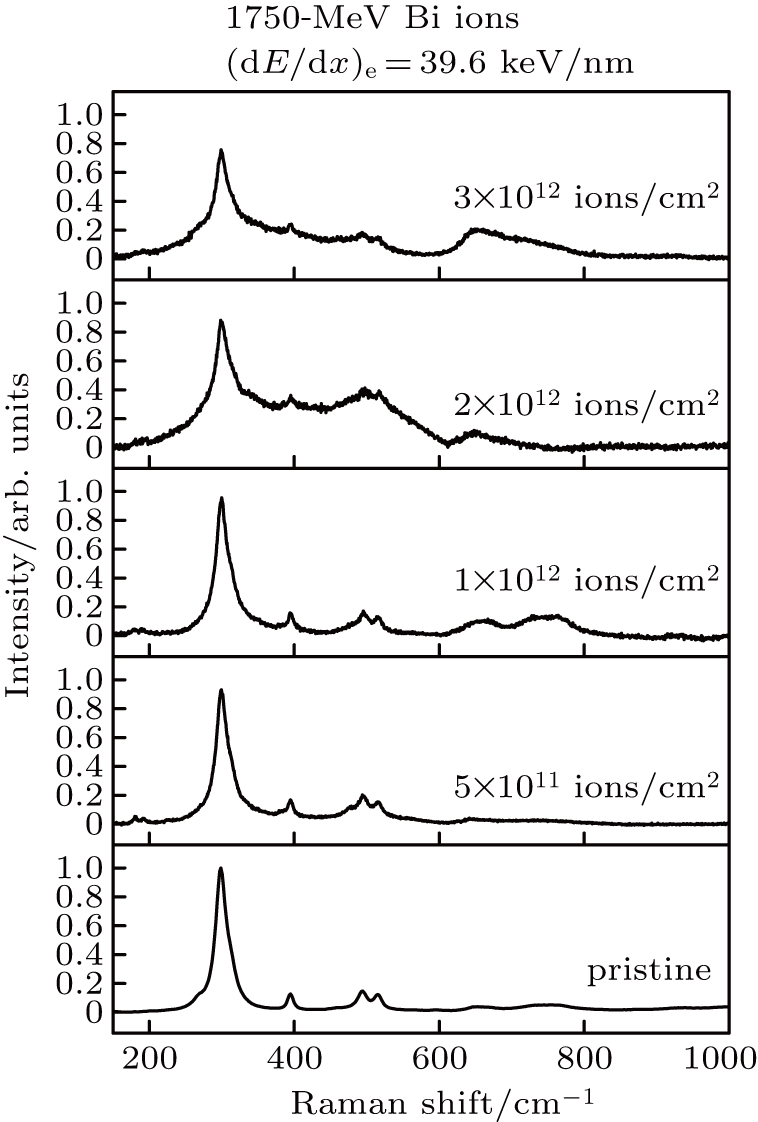 | Fig. 3. Plots of intensity versus Raman shift of La2Zr2O7 pyrochlore recorded before and after the irradiation of Bi ions with 39.6 keV/nm at various fluences. |
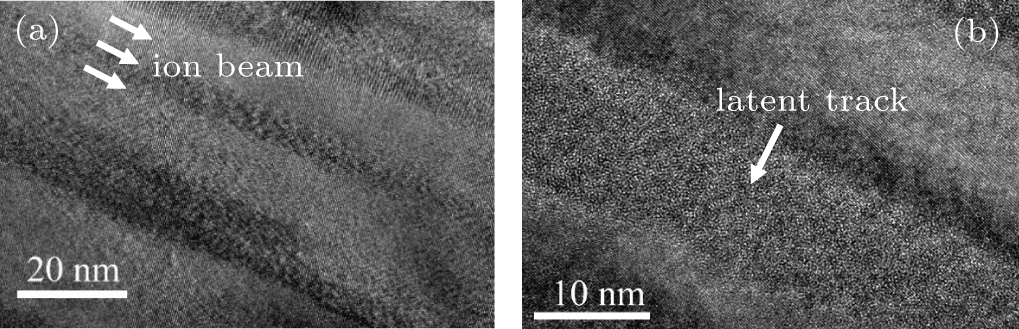
To observe the detailed microstructure of the irradiated pyrochlore, TEM is used to explore the pyrochlore irradiated by Bi ion with (dE/dx)e of 39.6 keV/nm. As expected, continuous tracks with amorphous structure in the core are found as shown in Fig.
In this wrok, La2Zr2O7 bulk samples are irradiated by various kinds of heavy ions (e.g., Ar, Kr, Bi) in a large energy regime in HIRFL to systematically study the vibrations and phase transitions in heavy ion irradiated pyrochlore. For La2Zr2O7 pyrochlore irradiated with (dE/dx)e of 16.0 keV/nm, infrared modes F1u at wavenumbers 192, 308, and 651 cm−1 appear in Raman spectra due to the irradiation induced atom displacement and octahedron deformation. The F2g band at 265 cm−1 rises up due to oxygen diffusion or adsorption caused by ion irradiation. For La2Zr2O7 pyrochlore irradiated with higher (dE/dx)e, e.g., 39.6 keV/nm, pyrohlore–amorphous transition occurs according to Raman study. The TEM measurements of Bi ion irradiated pyrochlore verify the generation of latent track with amorphous structure in the core.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] |