† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 51672029, 51372271, and 51172275) and the National Key Research and Development Project from the Ministry of Science and Technology, China (Grant No. 2016YFA0202702).
Among all-solid-state batteries, rechargeable Al-ion batteries have attracted most attention because they involve three-electron-redox reactions with high theoretic specific capacity. However, the solid Al-ion conductor electrolytes are less studied. Here, the microscopic path of Al3+-ion conduction of NASICON-type (Al0.2Zr0.8)20/19Nb(PO4)3 oxide is identified by temperature-dependent neutron powder diffraction and aberration-corrected scanning transmission electron microscopy experiments. (Al0.2Zr0.8)20/19Nb(PO4)3 shows a rhombohedral structure consisting of a framework of (Zr,Nb)O6 octahedra sharing corners with (PO4) tetrahedra; the Al occupy trigonal antiprisms exhibiting extremely large displacement factors. This suggests a strong displacement of Al ions along the c axis of the unit cell as they diffuse across the structure by a vacancy mechanism. Negative thermal expansion behavior is also identified along a and b axes, due to folding of the framework as temperature increases.
Limitations of the flammable liquid electrolyte of the Li-ion battery have motivated reconsideration of all-solid-state batteries.[1–7] Aluminum (Al) is an abundant, light-weight metal that offers three electrons per atom Al. Therefore, a theoretical specific capacity of 2980 mAh·g–1 and a volume capacity of 8046 Ah·L–1 can be achieved.[8,9] Recently, Dai et al.[10] reported a rechargeable Al battery with a three-dimensional graphitic-foam cathode that showed high-rate capability and excellent cycling performance up to 7500 cycles at room temperature. However, their cells used an ionic liquid electrolyte, AlCl3/[EMIm]Cl, that is costly and not easy to prepare. Development of an Al battery with a solid electrolyte is plagued by poor Al3+ conductivity, σAl, in the electrolyte. However, Imanaka et al. have reported a high σAl at high temperatures in (Al0.2Zr0.8)20/19Nb(PO4)3.[11]
In this work, we identify the Al diffusion mechanism in (Al0.2Zr0.8)20/19Nb(PO4)3 through high-temperature neutron powder diffraction (NPD) experiments and atomic-resolution scanning transmission electron microscopy (STEM) analyses. Temperature-driven Al displacements indicate that Al3+ ions diffuse across the structure by a vacancy mechanism. Furthermore, negative thermal expansion (NTE) behavior was also observed in (Al0.2Zr0.8)20/19Nb(PO4)3 over a wide temperature range by NPD experiments. Importantly, we have demonstrated a rechargeable solid-state Al battery with V2O5 nanorods/rGO (reduced graphene oxide) as cathode, a dense (Al0.2Zr0.8)20/19Nb(PO4)3 pellet as electrolyte and Al as anode. Preliminary results show that (Al0.2Zr0.8)20/19Nb(PO4)3 is an electrolyte material for a solid-state Al battery operating at high temperatures. High-temperature batteries are important power sources for aeronautics, astronautics, and military applications.[12–14]
(Al0.2Zr0.8)20/19Nb(PO4)3 powder was prepared by a solid-state reaction method. The stoichiometric amounts of Al(OH)3, ZrO(NO3)2, Nb2O5, and (NH4)2HPO4 (Alfa Aesar Co. Ltd ) were mixed for 1 h with an agate mortar and then ball milled for 6 h with ethyl alcohol as a medium. The mixed slurry was dried in an oven overnight at 60 °C to get the precursor and then they were calcined at 600 °C for 6 h. The obtained powder was pelletized under 12 MPa before they were transferred to a muffle furnace and heated at 1025 °C for 14 h in air with a heating/cooling rate of 5 °C/min. The pellets were then pulverized, ground and pelletized again before heated to 1225 °C, and then 1275 °C and 1325 °C for 14 h, respectively.
The V2O5 nanorods/rGO composite cathode was prepared according to the literature.[14]
X-ray diffraction (XRD) was performed with a Bruker-AXS D8 diffractometer (40 kV, 30 mA) with Cu Kα radiation (λ = 1.5418 Å). Neutron powder diffraction (NPD) data were collected in the D2B diffractometer at the Institut Laue-Langevin, Grenoble with λ = 1.594 Å. For the RT acquisition, about 3 g of sample was contained in a vanadium can. For the high-temperature acquisitions, the sample holder was placed in the isothermal zone of a furnace with a vanadium resistor operating under vacuum (PO2 ≈ 10–6 Torr, 1 Torr = 1.33322×102 Pa). The measurements were carried out at 200, 400, 600, and 800 °C, respectively. The collection time was 2 h per pattern. The diffraction data were all analysed by the Rietveld method with the FULLPROF program[15] and the use of its internal tables for scattering lengths. The line shape of the diffraction peaks was generated by a pseudo-Voigt function. The irregular background coming from the furnace was extrapolated from points devoid of reflections. In the final run, the following parameters were refined: background points, zero shift, half-width, pseudo-Voigt and asymmetry parameters for the peak shape, scale factor and unit-cell parameters. Two regions with peaks coming from the experimental environment were excluded from the refinement. Isotropic thermal factors for all the metal atoms and anisotropic for aluminium atoms were also refined for the NPD data. The coherent scattering lengths for Al, Nb, Zr, P, and O were, 3.449, 7.054, 7.160, 5.130, and 5.803 fm (1 fm = 10–15 m), respectively.
TEM samples were prepared from (Al0.2Zr0.8)20/19Nb (PO4)3 pellets using a Gatan precision ion polishing system (PIPS II). A double aberration-corrected microscope JEM-Z3100F-R005 STEM/TEM operated at 300 keV was employed to do EDPs, HRTEM and STEM analyses. HRTEM images were collected with a negative spherical aberration (Cs) coefficient ∼ –30 μm and slight over focus to obtain bright atomic images. An image simulation software JEMS was used to simulate HRTEM images with multislice computation method. The crystal structural model proposed by neutron diffraction analysis at 25 °C was used for the HRTEM image simulation. Atomic resolution HAADF-STEM images were collected with a probe convergence semi-angle of 24 mrad, and HAADF detector semi-angular collection range 42 mrad–240 mrad.
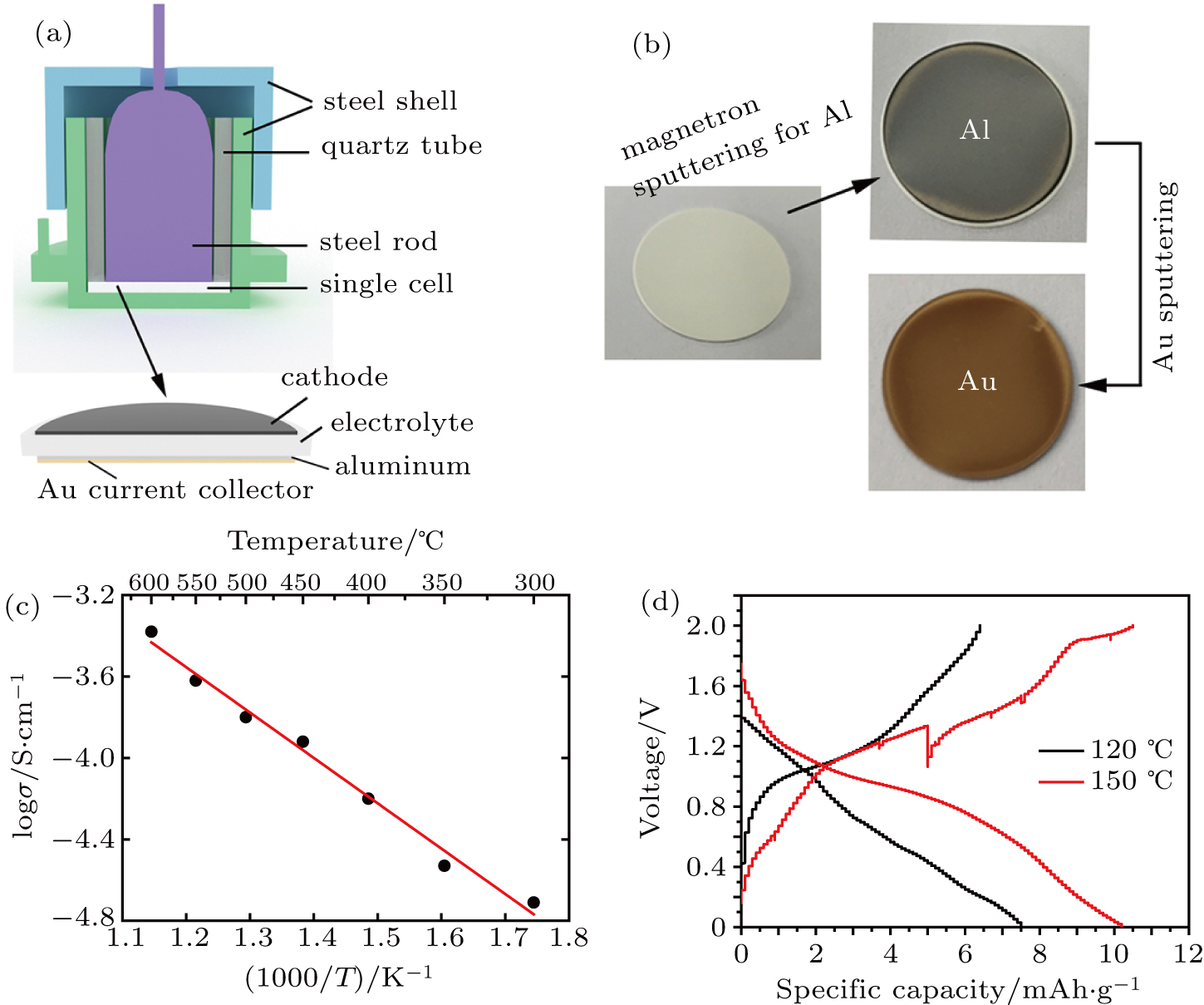
Electrochemical performance testing was performed with swagelok cells. The cells consisting of the V2O5 nanorods/graphene as cathode, aluminum as anode and the prepared dense (Al0.2Zr0.8)20/19Nb(PO4)3 solid pellet (∼ 300 μm in thickness, 13 mm in diameter) as electrolyte were assembled in an Ar-filled glove box. Typically, for anode preparation, aluminum was deposited on one side of the dense electrolyte pellet by a magnetron sputtering technique (Kurt J Lesker PVD75 Proline, USA) and subsequently sputtered Au coating as the current collector by a sputtering machine (Cressington 108 auto, UK).[16] For improving the ion diffusion issue between the cathode/electrolyte interface, a small amount of the molten-salts electrolyte consisting of sodium chloride (99.99%) and aluminium chloride (99.9%) (1:1.63 in mole ratio) was introduced, as reported in the literature.[17,18] The cathode area is 0.785 cm2 and the active mass of the cathode is about 2 mg·cm–2. Galvanostatic discharge/charge test was carried out in the voltage range of 0.01 V–2.0 V at a current density of 2 mA·g–1 at 120 °C and 150 °C on a LAND battery testing system, respectively.
For impedance spectra measurements, the obtained powder was pressed at 15 MPa into pellets. The pellets are 13 mm in diameter. The thickness of the pellets is about 1 mm. The pellets were then coated with platinum paste on both sides. AC impedance spectroscopy was measured with an electrochemical workstation (Zahner IM6E) over a frequency range from 100 mHz to 1 MHz. An applied voltage was fixed at 10 mV. All measurements were done in temperature range of 300 °C to 600 °C.
(Al0.2Zr0.8)20/19Nb(PO4)3 was prepared by solid-state reaction. The phase and crystal structure of the product were examined by powder x-ray diffraction (XRD); the pattern is shown in Supplementary Material, Fig. S1. All of the peaks of the well-crystallized structure correspond to the rhombohedral NASICON phase isostructural to NaZr2(PO4)3.[19,20] The octahedrally coordinated Zr4+ are partially replaced by Nb5+, which reduce the volume of the unit cell sufficiently to accommodate the small Al3+ (0.535 Å in octahedral coordination) in the interstitial space of the M(II) site of the structure.[21] In NaZr2(PO4)3, the Na+-ions occupy the interstitial M(I) sites, and in (Al0.2Zr0.8)20/19Nb(PO4)3, the M(I) sites are occupied by Nb and/or Zr. Our original strategy was to also increase the oxygen bonding of the framework to reduce the strength if the Al3+ is bonded to the framework.[11]
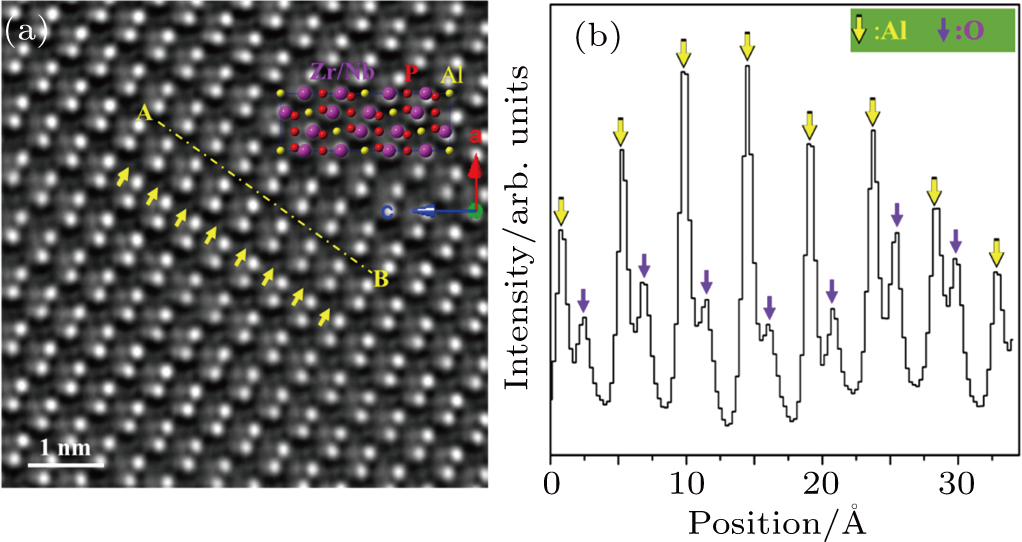
Electron-diffraction pattern (EDP) and aberration-corrected high-resolution transmission electron microscopy (HRTEM) taken at 25 °C were also performed to characterize the crystal structure. All of the EDPs and HRTEM images can also be well-indexed with a NASICON-type model(Supplementary Material, Fig. S2, Table S1). Atomic-resolution high-angle annular dark field (HAADF) imaging was performed with an aberration-corrected scanning transmission electron microscopy (STEM) to observe the Al3+ distribution. Figure
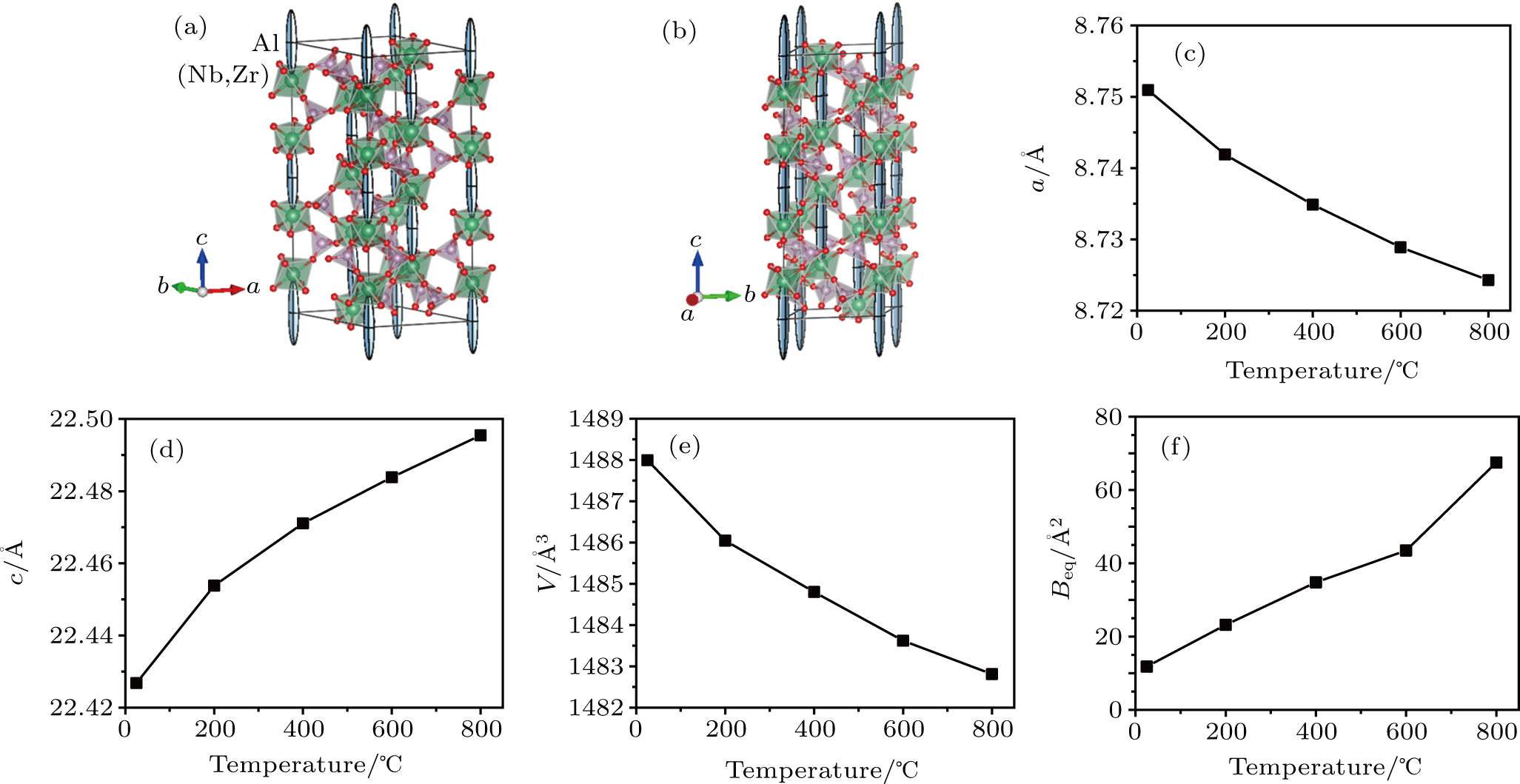
An investigation of the crystal structure by NPD was performed to determine structural details, since neutrons allow us to explore a much wider range of the reciprocal space and the lack of form factor permits the precise determination of the anisotropic displacement factors. The neutron pattern confirms the rhombohedral symmetry with a = b = 8.7509(2) Å and c = 22.4368(7) Å in the hexagonal setting at 25 °C; the structure was defined in the R-3c space group (No. 167) with Al located at 6b Wyckoff site (0,0,0); Nb and Zr distributed at random at 12c (0,0,z) site; P at 18e (x, 0, 1/4) positions and the two types of oxygen O at 36f (x,y,z) sites. The Nb and Zr occupancy factors were defined according to the nominal stoichiometry; the Al occupancy factor was also fixed to the nominal composition since it was found to be strongly correlated with its thermal displacement factors. The final structural parameters and agreement factors are gathered in Supplementary Material, Table S1.
Conductivity measurements, as described later, demonstrate that (Al0.2Zr0.8)20/19Nb(PO4)3 is an exceptionally good solid-state conductor for Al3+ ions. A temperature-dependent NPD study appears to be critical to understand the crystal structure evolution with the functional temperatures of this material. NPD diagrams collected at 200, 400, 600, and 800 °C with λ = 1.594 Å show the same crystal symmetry but dramatic changes in the magnitude and anisotropy of the displacement parameters. All the peaks can be indexed in the rhombohedral R-3c unit cell, which demonstrates the structure is stable at these working conditions. The access to a wide region of the reciprocal space allowed us a successful refinement of the anisotropic displacement factors of the aluminum atoms. The good agreement between observed and calculated profiles is displayed in the patterns tested at 25 °C and 800 °C, respectively (Supplementary Material, Figs. S3(a) and S3(b)). Figures
It is remarkable that whereas the c parameter expands upon heating, the a parameter contracts in all the studied temperature range (25 °C–800 °C), yielding an overall, anisotropic volume contraction as temperature is increased. The thermal expansion coefficient based upon the volume variation may be defined as αV = (VT – V0)/V0(T – T0), giving αV = –4.49×10–6 K−1. If we estimate the contraction experienced along the a or b direction, according to αl = (lT – l0)/l0(T – T0), we obtain αa = –3.92×10–6 K−1. The expansion along c is αc = 3.37×10–6 K−1. This anisotropic thermal expansion has been observed before in other NASICON-type structures.[22,23] The origin of the observed NTE has been previously described as a folding of the structure on warming up. The microscopic origin of the NTE is identified by our NPD data.
The magnitude of the thermal displacement increases monotonically with temperature, as is shown in Table S1 and Fig. S3. The anisotropy of the Al thermal ellipsoids has the largest thermal displacement along the c axis. At 800 °C, the root mean square (r.m.s.) displacements of Al are 1.56 Å along the [001] direction, and 0.1 Å and 0.01 Å perpendicular to it, in a flattened and extraordinarily elongated prolate configuration (Fig. S3). As discussed later, this configuration shows a strong displacement of the Al atoms, even penetrating into the adjacent (Nb,Zr)O6 octahedra that contain some vacancies at 800 °C for the nominal composition (Al0.2Zr0.8)20/19Nb(PO4)3, implying that 8% of the octahedral M(I) positions are empty. Diffusion by a vacancy diffusion mechanism is possible because only 41% of the 6b positions are occupied by Al atoms. This result is consistent with the HRTEM results. Given the rombohedral symmetry, a largely anisotropic Al diffusion would be expected for this compound, mainly along the c axis.
The feasibility of a dense (Al0.2Zr0.8)20/19Nb(PO4)3 pellet as a solid electrolyte material was investigated by impedance spectra measurements. The cross sectional SEM images of the prepared (Al0.2Zr0.8)20/19Nb(PO4)3 pellet are shown in Fig. S4. Swagelok cells were prepared with V2O5 nanorods/graphene as cathode,[24] aluminum as anode and the dense (Al0.2Zr0.8)20/19Nb(PO4)3 solid pellet (∼ 300 μm in thickness) as electrolyte. Figure
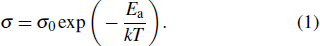 |
The (Al0.2Zr0.8)20/19Nb(PO4)3 crystal structure is a three-dimensional (3D) network containing vertex-linked (Nb,Zr)O6 octahedra and PO4 tetrahedra, with the Al-ions partially occupying interstitial trigonal antiprism spaced at intervals along the c axis. There is only one crystallographically distinct Al site. Figure
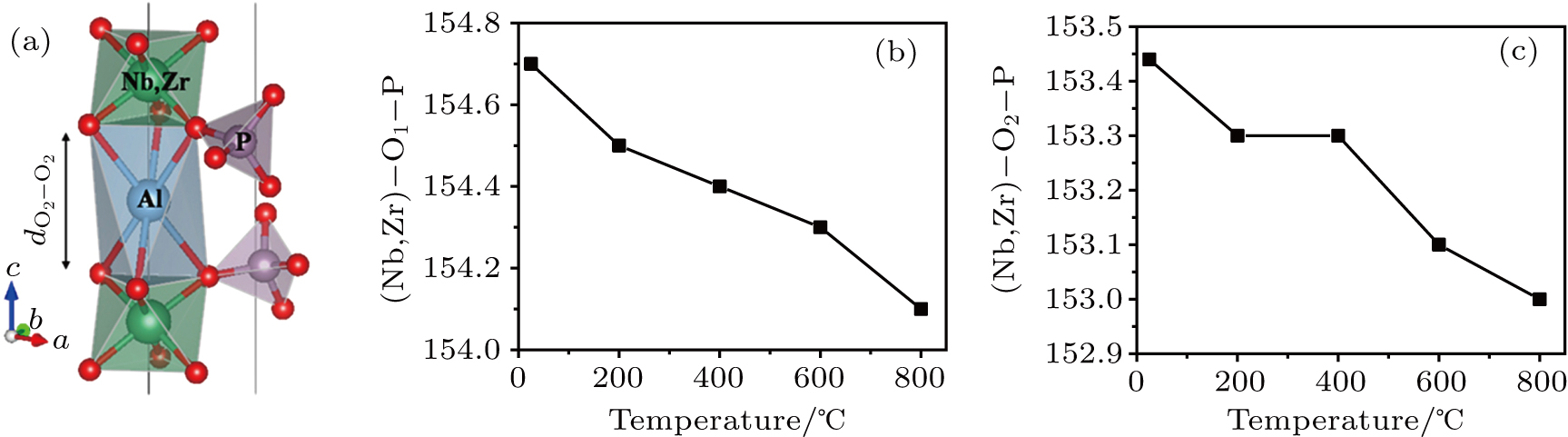
The thermal expansion of the c axis observed in this temperature range may be explained by the expansion of the c-axis O2–O2 separation of the Al site. This governs the ‘size’ of the trigonal prismatic site along the c axis. Between 25 °C and 800 °C the O2–O2 distances along the c axis (dO2–O2 in Fig.
This NTE behavior is a general trend in many frameworks containing octahedral and tetrahedral units, such as ZrW2O8,[24,25] ZrMo2O8,[26] and other NASICON-type structures including the related Al-free NbZr(PO4)3 with (αl = 2.3×106)[27] and (HfSc)0.83W2.25P0.83O12δ.[28] This behavior is a consequence of the structural constraint of atomic bridging motifs.[26] The ability to bend these linkages at the O atoms means that the dominant thermal response is associated with flexing bonds rather than stretching them, and the geometrical flexibility explains why the crystallites deform readily in response to thermal and/or mechanical stress in NTE materials.
The high Al3+conductivity is primarily linked to the weak chemical bonds established between Al and O2 in an over-sized cage exhibiting Al–O2 distances in the 2.64 Å–2.65 Å range. The ionic radii sums for Al3+ (i.r.(VI) = 0.535 Å) (16) and O2– (i.r.(IV) = 1.38 Å) is just 1.915 Å, meaning that Al ions are rattling in the cages, as is visualized by the large displacement factors observed even at room temperature. It is noteworthy that the orientation of the anisotropic thermal displacements is along the axial directions of the trigonal antiprism; i.e., approximately along the bonding direction within the octahedra. This arrangement of the thermal ellipsoids is not the one usually found in oxides; normally the thermal vibrations are perpendicular to the chemical bonds, displaying oblate spheroids. Taking the cubic perovskite Ba0.87K0.13BiO3[27] as an example, the oblate vibrations lock the oxygen atoms at mid-bismuth positions with enhanced vibrations perpendicular to the Bi–Bi distance. A prolate ellipsoid for the oxygen anisotropic thermal displacement parameters indicates a softening of “breathing” modes at the bismuth atoms. In the present (Al0.2Zr0.8)20/19Nb(PO4)3 phosphate, the prolate thermal ellipsoids of the Al3+ ions are oriented along the chemical bonds and are clearly correlated with the Al diffusivity.
In the present investigations, the NASICON-type oxide (Al0.2Zr0.8)20/19Nb(PO4)3 is a good solid-state conductor for Al3+ ions. Temperature-dependent neutron powder diffraction (NPD) and aberration-corrected scanning transmission electron microscopy (STEM) experiments identify a strong displacement of Al ions along the c axis of the unit cell as they diffuse across the structure by a vacancy mechanism. The Al3+ conductivity and NTE of the (Al0.2Zr0.8)20/19Nb(PO4)3 may be applicable in wide applications, such as solid-state Al batteries and precision optical devices used on satellites.[29]
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] |